Business Editors/Health/Medical Writers
INDIANAPOLIS--(BUSINESS WIRE)--Oct. 8, 2003
Lilly Initiative Provides Investigational Agent Free of Charge
for Medically Eligible Patients with Mesothelioma
In just over a year, 1000 patients in the United States diagnosed with malignant mesothelioma -- an asbestos-related cancer -- have received Eli Lilly and Company's investigational drug Alimta(R) (pemetrexed) as part of an expanded access, or compassionate use, program. The program, which was agreed to by the U.S. Food and Drug Administration (FDA) in May 2002, provides a treatment option for patients diagnosed with mesothelioma, prior to a regulatory decision on the drug.
"This is extremely gratifying," said Paolo Paoletti, M.D., vice president of oncology products at Eli Lilly and Company, who added that an additional 1,075 patients with malignant mesothelioma have been enrolled in the program outside of the United States. "We are not only prolonging the survival of patients but also making them feel better."
Malignant mesothelioma, a rare form of cancer, is a disease in which cancer cells are found in the sac lining the chest (the pleura), the lining of the abdominal cavity (the peritoneum) or the lining around the heart (the pericardium). By the time symptoms of mesothelioma appear, the disease has usually progressed to an advanced stage.
Expanded access, or "compassionate use," programs are designed to make investigational agents like Alimta accessible as quickly as possible to treat patients with diseases for which no comparable or satisfactory alternative drug or other therapy is available.
"When I open my eyes each morning, I thank goodness for the day I enrolled in the Alimta expanded access program," said 55-year-old Richard Shanas, a patient with malignant pleural mesothelioma who has been in the program for six months. "Now, I look forward to each day and cherish the truly wonderful moments I am able to share with my wife and son."
The FDA agreed to the Alimta expanded access program based on a preliminary review of results from clinical trials evaluating the agent in mesothelioma. Included in this review were the results of a Phase III trial recently published in the Journal of Clinical Oncology.
Those findings showed that patients with mesothelioma treated with a combination of Alimta and cisplatin survived an average of 30 percent longer and experienced less pain and shortness of breath than those treated with cisplatin alone. In this trial, the most common side effect associated with Alimta and cisplatin was a decrease in infection-fighting white blood cells (technically known as neutropenia), though the rate of infection was very low.
"Alimta has given me a good quality of life," added Shanas. "While there are side effects with any chemotherapy, the side effects I have experienced while on Alimta have been mild in comparison to other chemotherapy drugs I have been on in the past."
Under the expanded access program, Alimta is available free of charge to patients with mesothelioma who meet medical eligibility criteria. For more information on the expanded access program for mesothelioma, physicians may call 1-866-347-9503 (patients are asked to work through their physicians). Additionally, information on this trial can be found at www.ClinicalTrials.gov, a service of the National Institutes of Health.
Lilly is currently pursuing FDA approval for Alimta in combination with cisplatin for the treatment of patients with malignant pleural mesothelioma and has completed its rolling submission to the FDA. The company and the FDA have also agreed on plans for Lilly to submit Alimta for second-line non-small cell lung cancer by the end of 2003. In countries outside of the U.S., including the European Union, Lilly is proceeding with Alimta submissions for both malignant pleural mesothelioma and second-line non-small cell lung cancer.
Alimta is also being evaluated in breast, ovarian, bladder and head and neck cancers, as well as gastrointestinal cancers of the stomach, pancreas and colon.
Lilly, a leading innovation-driven corporation is developing a growing portfolio of best-in-class pharmaceutical products by applying the latest research from its own worldwide laboratories and from collaborations with eminent scientific organizations. Headquartered in Indianapolis, Ind., Lilly provides answers -- through medicines and information -- for some of the world's most urgent medical needs. Additional information about Lilly is available at www.lilly.com.
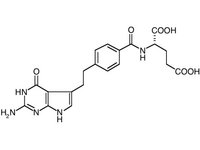
Alimta(R)(pemetrexed disodium, Lilly)
COPYRIGHT 2003 Business Wire
COPYRIGHT 2003 Gale Group