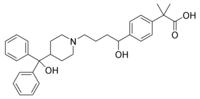
Barr Pharmaceuticals, Inc., recently received FDA approval to market a generic version of Allegra 60 mg. The company initially filed an application with the FDA in 2001, which lead to Aventis Pharmaceuticals initiating a lawsuit, citing patent infringement. Although Barr Pharmaceuticals has received FDA approval, it plans to delay marketing the generic version of Allegra 60 mg as it awaits a patent trial to commence in early 2006.
COPYRIGHT 2005 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2005 Gale Group