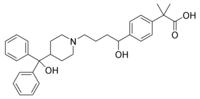
A 24-hour extended-release tablet form of fexofenadine HCl (Allegra-D 180 mg) made by Aventis Pharmaceuticals, Inc., was approved by the FDA for the relief of symptoms associated with seasonal allergic rhinitis in adults and children aged 12 years and older. This formulation was developed with aaiPharma using a patented extended release drug delivery technology, ProSlo II[TM] developed by Osmotica Pharmaceuticals. Aventis currently markets twice-daily Allegra-D (fexofenadine HCl 60 mg/pseudoephedrine HCl 120 mg) extended release tablets. Allegra-D 24 Hour is the only approved once daily prescription antihistamine with a decongestant.
COPYRIGHT 2005 Journal of Drugs in Dermatology
COPYRIGHT 2005 Gale Group