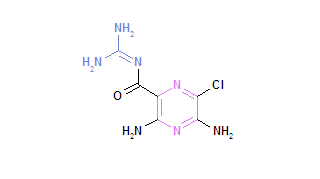
Insights into airway epithelial ion transport, an important component of lung defense, have recently heralded a new era in lung research, and novel treatments are also being developed to modulate these systems.[1,2] Cystic fibrosis (CF) represents a prototype disease, associated with defects in [Na.sup.+] and [Cl.sup.-] transport.[3] Current treatments for CF target the secondary manifestations of the basic pathophysiologic defect, and it is imperative that new treatments continue to be developed.[4] Agents that address the basic defects in ion transport in CF airways include the selective [Na.sup.+] channel blocker amiloride[5,6] and uridine-5'-triphosphate (UTP), drugs that modulate ion transport, and are delivered by the aerosol route.[7,8]
To target ion transport defects, aerosol delivery of the drugs is required for local airway action. A variety of nebulizers are available for such aerosol therapy, including continuous flow (compressed air) jet nebulizers and ultrasonic nebulizers.[9-11] The efficiency of nebulizers to deliver pulmonary drugs varies between machines, reflecting different rates of outputs, the aerosol size distribution, the flow of the gas driving jet nebulizers, and the volume of fill.[9,10,12] Other factors influence aerosol deposition, including the properties of the liquid to be nebulized, lung function, and inspiratory flow rates of the subjects.[13]
A jet nebulizer previously used to deliver amiloride[14] to the airways of CF patients may have disadvantages from the point of view of design, aerosol droplet size, and output.[15,16] An ultrasonic machine (Omron NE-U07; Omron Healthcare; Vernon Hills, Ill) may offer advantages in terms of droplet size and output. We sought to define the concentration of amiloride on airway surfaces following aerosolization via this device. We used a novel technique, ie, filter paper sampling of airway surface liquid by a transbronchoscopic protected catheter.[14] This approach would provide direct information about drug deposition and clearance on proximal airways, which would allow estimates of the duration of action of amiloride on its target surface and calculation of dosing frequencies. In addition, we obtained a pharmacokinetic profile of the delivered drug by measuring amiloride in plasma and urine over time after aerosolization.
MATERIALS AND METHODS
Study Subjects
Eight normal subjects without any history of respiratory or allergic disease (mean age, 25.3 [+ or -] 1.4 years; range, 22 to 33 years) were screened and included in the study (data from one subject were excluded from final analyses; see below). Subjects had normal pulmonary function test results ([FEV.sub.1]=107.0 [+ or -] 4.7% predicted) and a normal chest radiograph. Informed consent was obtained under the auspices of the Committee on the Protection of the Rights of Human Subjects of the University of North Carolina at Chapel Hill.
Aerosol Solution and Delivery of the Drug
Amiloride hydrochloride (a gift of Merck, Sharpe and Dohme; Bluebell, Pa) was dissolved in 0.12% saline solution to a final concentration of 1 mg/mL (3.3x[10.sup.-3] mol/L), and 4.5-mL volume (measured by a 5-mL syringe) was placed in the medication cup of an ultrasonic nebulizer (Omron NE-UO7). Care must be taken while filling the medication cup with any sharp instruments, such as a syringe and needle, as the plastic cup is vulnerable to puncture. Subjects were instructed in a defined pattern of breathing.[14] In brief, subjects inhaled slowly over approximately 2 to 4 s through the mouthpiece, from functional residual capacity to total lung capacity, with a breath-hold for 2 to 3 s, followed by slow exhalation to functional residual capacity, over approximately 3 to 4 s, activating the nebulizer only during inspiration. Each subject breathed in this manner in the seated position for 12 min at approximately 6 to 8 breaths/min, without difficulty. Pilot experiments with the nebulizer over various times showed that 3.5 mL of the 4.5-mL nebulizer fill was discharged over 12 min in a linear fashion, and that a 1-mL "dead volume" was more slowly dispersed over another 5 to 7 min. We therefore chose 12 min to aerosolize the amiloride. The machine must be kept flat while aerosolizing, preferably on a firm surface to ensure rapid dispersion. After nebulization, the subjects rinsed their mouths with water, and spit out the rinse, to minimize drug remaining in the oral cavity. The volume of medication aerosolized was calculated by weighing the nebulizer before and after nebulization.
Airway Surface Liquid Sampling
After aerosolization, subjects underwent bronchoscopy in the prone position. IV midazolam (2 to 5 mg), atropine (0.5 mg), and meperidine (25 to 50 mg) were administered. Lidocaine (Xylocaine) gel (1%) was applied to the nasal passage, and 1 to 2 mL of lidocaine liquid (1%) was applied to the vocal cords under direct bronchoscopic visualization. No anesthetic was administered below the cords. Two subjects had sufficiently narrow nasal passages so that the bronchoscope had to be passed transorally. A sterile catheter (1.8-mm outside diameter; American Edwards; Santa Ana, Calif) containing a standard biopsy forceps gripping a pledges of filter papers (five preweighed 1.5 cm x 1.5 mm strips; Grade 541; Whatman International Ltd; Maidstone, England), triply washed in distilled water and dried, was advanced through the biopsy channel of the bronchoscope. The filter papers were protected from contamination during passage through the biopsy channel by a sterile plug inserted in the end of the catheter (Fig 1A). At the appropriate endobronchial location, the forceps was advanced from the catheter, and the pledget of filter papers was held in contact with the airways for 20 s (Fig 1B,C). Each individual pledget was withdrawn from the bronchoscope after sampling, for subsequent weighing and storage. Airway surface liquid was collected separately from several sites in the proximal airways--main bronchi, upper lobe bronchi, bronchus intermedius. Triplicate samplings (ie, three separate samples) were clustered as closely as possible around the 15-, 35-, and 55-min time points after aerosolization, with the bronchoscope held in the trachea between sampling times. The sites of sampling to distinguish possible sites of "pooling" of amiloride. Coughing occurred in some subjects, with resultant minor trauma to the airway mucosa, and minor bleeding; any contamination of filter paper with blood was recorded. To allow calculation of the volume of airway surface liquid recovered, the preweighed filter papers were weighed at 15-s intervals for 2 min postsampling, and the wet weight (ie, weight at the time of sampling) was calculated using linear regression. Wet weight minus dry weight provided the volume of airway surface liquid sampled. The pledgets were frozen at -20 [degrees] C in sealed plastic scintillation vials, pending amiloride analysis.
Blood and Urine Sampling
Blood was drawn from each subject into a lithium heparin tube at 0, 15, 30, and 60 min, and 2, 3, 4, 6, 8, 12, 24, 48, and 72 h (time 0=commencement of nebulization). Plasma was separated by centrifugation and the samples were frozen at --20 [degrees]C. until amiloride analysis was performed. Urine was collected preaerosolization, and from 0 to 2, 2 to 4, 4 to 6, 6 to 10, 10 to 24, 24 to 48, and 48 to 72h after aerosolization. The total volume of urine collected in each time interval was recorded, and a 10-mL aliquot was frozen at -20 [degrees] C for subsequent amiloride analysis.
To evaluate the site of early absorption of the amiloride lung surfaces vs oropharyngeal surfaces), four normal subjects gargled 0.08 mg amiloride every minute for 12 min on buccal surfaces and then swallowed, to mimic as closely as possible the nebulization process in terms of total maximum dose delivered (0.96 mg) and exposure time (0.96 mg was the highest and amiloride dose delivered to one of the subjects; this was judged the best dose to test the hypothesis that amiloride might be absorbed across oropharyngeal surfaces). This dose actually delivered to the subjects was estimated from total amiloride amiloride excretion over 72 h, this estimation being possible since amiloride is excreted unaltered, largely through the kidneys.[14] Blood was collected at 0, 15, 30, and 60 min, and 2, 3, and 4 h postcompletion of the experiment, and plasma samples were stored at -20 [degrees] C for subsequent analysis of amiloride.
To ensure no detrimental effect of ultrasonic nebulization on amiloride, we performed a dry run, nebulizing an identical preparation of 4.5 mL of amiloride (1 mg/mL, 3.3 x [10.sup.-3] mol/L through the nebulizer (Omron), and taking samples from the cup prior to nebulization, at 3 min into nebulization, postnebulization, and also from the aerosol cloud by condensation into a plastic container. The samples were analyzed by high-performance liquid chromatography (HPLC). We characterized the particle size of the aerosol cloud produced by the nebulizer (using a Malvern Mastersizer-X; Malvern, United Kingdom), using the same concentration of amiloride as in the study (1 mg/mL). For comparison, we repeated the analysis under similar conditions for the jet nebulizer used previously for amiloride delivery (Devilbiss 646; Somerset, Pa), at a driving airflow of approximately 9 L/minn.
Analysis of Samples
The filter papers were analyzed for the mass of amiloride (by Glaxo Inc; Research Triangle Park, NC). Briefly, each pledget was placed in a vial; 2 mL methanol and 200 mL triamterene in methanol (200 ng/mL; Sigma; St. Louis) were added as the internal standard, and the vial was mixed for 3 min. The contents of the vial were transferred into a culture tube, and the solvent was evaporated at 37 [degrees] C under nitrogen. The residue was dissolved in 1 mL of mobile phase, and 30 [micro] L was injected into a HPLC system as previously described.[17] The mass of amiloride was determined in nanograms per pledget of filter papers, and the concentration of amiloride was calculated. The amiloride samples from the nebulization dry run experiment were diluted and injected into a similar HPLC system in our laboratory. Full details of the assay performance characteristics, precisions, and accuracy are outlined by Selinger.[17] The within-run precision and accuracy were 2 to 2.2% and 97.8 to 103.7% respective.
The plasma and urine samples were analyzed (by Cedra Corp; Austin, Tex) in a methods similar to that described above. Aliquoats were mixed with internal standard triamterene (15 ng/mL), and the amiloride peaks were analyzed by HPLC. The range of quantitation for the assay was 0.5 to 40 ng/mL, with the mean reproducibility of the test being 93.6 to 106% when several replicates of quality controls were tested against three quality control samples previously prepared in serum (1, 15, and 30 ng/mL). Data are expressed in nanograms per milliliter for plasma, and total amount (micrograms) amiloride excreted in urine.
Data Analysis
The data from one subject were excluded from further analysis due to leakage of the medication cup during nebulization--the plastic medication cup was inadvertently punctured during placement of the drug with a syringe and needle, resulting in minimal nebulization of drug.
A mixed model analysis[18] was used to assess the relationship between airway surface liquid amiloride concentration (outcome variable) and predictors to address whether the data fit a curvilinear or a straight linear regression plot: elapsed time, sampling site (dependent vs nondependent), and contamination (blood vs nonblood contaminated). To address whether the data supported a curvilinear fit, we used a polynomial model to test for the statistical significance of the quadratic component (time-squared) A model using sampling site, contamination, and linear and quadratic time was initially fit to the data. The result showed that the quadratic component in the model did not explain a significant amount of the variability in log molarity (de, the data did not fit a curvilinear model). A reduced model using sampling site, contamination, and linear time showed that only the slope (and intercept) were statistically significant (p [is less than] 0.05). The interactions between linear time and sampling site and contamination were not statistically significant. We concluded that the data best fit a linear model in time.
The pharmacokinetic data (mean [+ or -] SEM) were plotted as plasma amiloride concentrations vs time, and urinary excretion of amiloride (percent of total) during each collection interval. Pharmacokinetic modeling was employed to address the absorption processes across lung surfaces vs oropharyngeal surfaces. Three models were fit to the amiloride plasma concentration-time data from the present study, using data from six of the subjects, since the data from one subject did not have enough time points to satisfy the modeling analysis: (A) model 1 assumed that amiloride was absorbed only from site 1 (presumably the lung) through time T (where T = the time taken for amiloride to be absorbed across site 1). (B) model 2 assumed that amiloride was absorbed only from site 2 (presumably the GI tract), with a lag time prior to absorption, and (C) model 3 assumed that drug was absorbed only from site 1 through time T, and subsequently from site 2. Three criteria were used to select the "best fit model:" visual inspection of the simulated vs the observed data, the rank and condition number of the matrix of the partial derivatives, and Akaike's Information Criteria.[19]
RESULTS
In the dry-run experiment, four HPLC chromatograms were generated from the samples collected (data not shown). The amiloride peak occurs at approximately 8 min with the same characteristics in all four chromatograms, and eluted at the same time as a known standard, thus confirming that amiloride is chemically unaltered by the process of ultrasonic nebulization. Using the nebulizer, the mass median aerodynamic diameter (MMAD) of the particles was 4.88 [micro] m, With 54% of the droplets in the respirable range (1 to 5 [micro]m). Using the jet nebulizer (DeVilbiss 646), the MMAD was 4.98 [micro]m, with 47% of the particles in the respirable range.
The mean volume aerosolized was 3.5 [+ or -] 0.3 mL during 12 min of aerosolization time, which is 77% of the dose of amiloride originally in the nebulizer. The mean dose systemically absorbed by the study subjects, as determined by the urine output of amiloride over 72 h, was 0.63 [+ or -] 0.07 ma, which is 14% of the original mass of drug.
Eighty-seven percent of the absorbed amiloride was excreted in the urine within the first 24 h. No amiloride was detected in the urine of any subject after 48 h (Fig 2). The volume of urine collected for all subjects over the 72 h was 4.3 [+ or -] 1.1 L (range, 3.3 to 8.3 L).
[Figure 2 ILLUSTRATION OMITTED]
The log concentration of amiloride in airway surface liquid vs time is depicted in Figure 3. After applying the mixed model analysis as outlined above, linear regression was used to estimate amiloride concentration in airway surface liquid after nebulization at "time = 0" (1.6 x [10.sup.4] mol/L), and the half-life for elimination (approximately 23 min).
[Figure 3 ILLUSTRATION OMITTED]
After aerosol delivery, amiloride plasma concentrations (Fig 4) increased rapidly to an apparent peak concentration at 30 min (3.36 [+ or -] 0.72 ng/mL). Only one subject had measurable amiloride concentrations after 24 h. In contrast, no amiloride was detected at 30 min in the plasma of any of the four subjects after amiloride was swished and swallowed, suggesting that absorption across oropharyngeal surfaces does not contribute to this early amiloride concentration. The simulated amiloride concentration time profiles based on models 1, 2, and 3 are shown in Figure 5. Early (30 min) peak concentrations of amiloride were consistent with the model that assumed only lung absorption through T = 0.62 h (model 1). The amiloride plasma concentration-time profile through T = 12 h was best described by the model that assumed only lung absorption from 0 to 0.62 h, and only absorption from the GI tract after 0.62 h (model 3). These models support the hypothesis that the early peak plasma concentration in Figure 4 reflects absorption across lung surfaces, with later concentrations reflecting absorption across GI surfaces.
[Figure 4-5 ILLUSTRATION OMITTED]
DISCUSSION
The past decade has seen exciting advances in our understanding of airway epithelial ion transport, an important system that contributes to mucociliary defense mechanisms.[1,2] CF is a disease that is characterized by abnormalities in airway epithelial ion [Na.sup.+] and [CI.sup.-] transport.[20] New therapies that target these abnormalities are being tested, and show promise, including amiloride and UTP.[7,8,21] Since amiloride acts in a concentration-dependent manner to inhibit [Na.sup.+] channels,[22] evaluation of aerosol delivery to the airway must include measures of airway surface liquid concentrations and pharmacokinetics. We evaluated delivery of amiloride to the airway surfaces of normal individuals via an ultrasonic nebulizer that produces an aerosol cloud with 54% of the droplet size in the respirable range, and an MMAD of 4.88 [micro] m. This was achieved by directly measuring the concentration of amiloride deposited on airway surfaces. Since the effect of amiloride is dose dependent, this outcome measure is a direct indicator of a therapeutic effect.[22] Using a specific breathing pattern to optimize deposition, this study demonstrates that a dose of amiloride can be delivered to proximal airway surfaces via this ultrasonic nebulizer, and achieve a concentration that fully inhibits [Na.sup.+] transport in airway epithelia.[23] The ranges of concentrations achieved in the study indicate initial airway surface liquid amiloride concentrations of approximately 1.6 x [10.sup.-4] mol/L and an elimination half-life on airway surfaces of approximately 23 min. It is known that the concentration of amiloride in airway surface liquid required to produce effective blockade ([is greater than] 90% inhibition) of the absorption of sodium in normal subjects and in patients with CF is approximately [10.sup.-5] mol/L (the dose required for 50% of maximal effect is [10.sup.-6] mol/L).[1,2,22,24,25] Thus, an adequate concentration of amiloride for inhibition of [Na.sup.+] transport is present on proximal airway surfaces for approximately 3 to 5 h after delivery via the ultrasonic nebulizer.
It should be noted that the pattern of early disposition (ie, within the first 20 min) is especially difficult to quantify, since dosing took 12 min, and the earliest samples were obtained 10 to 15 min later. The precise pattern of elimination during this time period is not clear, but clearance appeared to be first order over the time that amiloride samples were obtained.
The systemic pharmacokinetic data support the concept that the ultrasonic nebulizer delivers drug to the lung. The peak early (30 min) plasma level (Fig 4) is most likely the result of early absorption across lung surfaces (see below), and there is no later (3 to 4 h) peak that would reflect further absorption from the GI tract. In previous studies, peak plasma concentrations after an oral dose of amiloride were observed consistently at approximately 4 h after administration.[14] Aerosolization of a similar dose of amiloride via a jet nebulizer resulted in two peak plasma concentration levels, one at 30 min, and a later peak at 3 to 4 h; the second peak concentration likely represents GI absorption of amiloride after oropharyngeal deposition and swallowing.[26,27] Complementary data to suggest the early (30 min) maximum plasma concentration observed in this study reflects absorption via the lung include the following: (1) there was no measurable plasma amiloride at 30 min in subjects who swished and swallowed an equivalent dose of amiloride, which suggests that oropharyngeal surfaces are not significant routes for absorption of amiloride, and (2) the pharmacokinetic modeling demonstrated that the early peak concentrations correlated with the model that assumed absorption via the lung; the entire amiloride concentration-time profile was best described by a model that assumed only lung absorption through time T, and subsequently only GI absorption.
Although we limited the measurements of amiloride to proximal airways sites in this study, deposition at distal airway sites may be comparable to that of other studies, recognizing that these studies are technically challenging and the study population is small. Previous studies in children with CF using a jet nebulizer demonstrated that amiloride concentrations (corrected for dilution) in bronchial wash specimens from distal airways correlated with direct measures at proximal sites.[28] One might expect that deposition in diseased airways, such as in those with CF, might not be as good as in normal subjects. However, previous studies involving identical sampling techniques (transbronchoscopic, as in this study) and formulation of drug in a jet nebulizer (DeVilbiss 646; 1.5 mg/mL; 5 x [10.sup.-3] mol/L) showed that comparable concentrations of amiloride were deposited on proximal airway surfaces of CF patients (n=10) and normal subjects (n=6), and the rate of clearance was similar.[14] These data suggest that comparisons can be drawn between delivery to CF and normal subjects.
The concept of assessing pulmonary bioavailability by measuring serum or urine samples has been advanced previously for salbutamol (albuterol) and nedocromil.[29,30] The relative bioavailability of salbutamol in the lung after inhalation was estimated from a 30-min urine sample,[29] and correlated with the amount of drug inhaled and deposited in the lung. The quantity of nedocromil deposited in the lungs postnebulization is the major determinant of the plasma drug concentration.[30] The data in the present study are consistent with the concept that a 30-min plasma sample could be used as an index of amiloride delivered to the lung, which would be a convenient measure of the efficiency of delivery devices and patient compliance with dosing schedules.
Various techniques have been used to test delivery of drugs to the lungs.[11,31-33] An estimate of drug deposition to the airways can be made by measuring the concentration of aerosolized antibiotic in sputum following nebulization.[32-34] The dose of drug delivered can be calculated by using a radio-aerosol to label the nebulizer cloud, and utilizing gamma scanning to measure deposition in the lung.[31,33,35] The more direct method of sampling airway surface liquid, via the transbronchoscopic technique, to measure drug concentrations provides another way to assess the ability of a nebulizer to deposit medication in the airways.[14] This is particularly relevant in the case of drugs that target ion transport on the airways, and exert an effect in a concentration-dependent manner, such as amiloride. Other drugs that modulate airway epithelial ion transport are currently being developed, for example, newer analogues of amiloride (benzamil) or chloride secretagogues (UTP).[7,8,36,37] Sampling and estimation of airway surface liquid have been addressed previously using a variety of techniques, including the urea dilution method, but it is not straightforward.[38-41] A previous study using a jet nebulizer and amiloride[14] showed that transbronchoscopic collection of airway surface liquid with filter papers housed in a sterile catheter is feasible. A similar study in children also measured amiloride in airway surface liquid via this technique, and in addition, as outlined above, showed that good correlation was achieved between amiloride concentrations in proximal airways and in samples obtained from a quick bronchial wash, reflecting deposition in more distal regions.[28] Although the measurements in this present study were limited to proximal airways, the previous study in children supports the suggestion that airway surface liquid concentrations of amiloride in distal airways are comparable. It is clear that amiloride will be most effective if predominantly delivered to the airways, the major target in the treatment of CF, in terms of hydration of airway secretions and enhancement of airway clearance. Drug deposition in the airways may be somewhat lower in CF patients, as demonstrated in previous studies,[14] but even allowing for this, amiloride concentrations in airway surface liquid are adequate to inhibit [Na.sup.+] transport on the airway epithelium for up to 3 to 5 h, particularly if patients with milder disease are the target population.
One feature of this ultrasonic nebulizer is useful for delivering a heat-sensitive drug such as amiloride: the medication sits in a water bath, that separates it from the piezoelectric transducer that generates the ultrasonic waves that ultimately create the aerosol. Our HPLC analysis showed that the amiloride is unaltered following the nebulization process with this device. However, this design also has disadvantages. There is potential for puncture of the medication cup if filling with a sharp instrument (such as a syringe and needle). It is also necessary to nebulize on a flat surface to ensure rapid even delivery of medication from this machine. Patients using any nebulizer for drug delivery need to be educated in the correct breathing technique, such as used in this study, to improve deposition of drug in the lung.[14]
Amiloride alone may be insufficient to exert a significant clinical effect on CF patients with established airways disease, but it may be useful in younger patients with CF, prior to significant airways disease.[42] Because the nature of the ion transport defects in CF is complex,[1,2] therapy other than amiloride may be necessary. In this context, UTP shows promise.[7,8] UTP has several actions on airway epithelia, including stimulation of ciliary beat frequency, degranulation of goblet cell mucins, and stimulation of [Cl.sup.-] secretion via alternative chloride channels on the apical surface of airway epithelial cells. Preliminary studies suggest that aerosolized UTP (alone or in combination with amiloride) improves whole lung mucociliary clearance in normal subjects, and peripheral lung mucociliary clearance in CF patients. Longer-acting analogues of amiloride (benzamil and phenamil) are also currently under scrutiny.[36,37] Finally, drugs that modulate ion transport may be useful in other diseases with impaired clearance of airway secretions; for example, aerosolized UTP improves whole lung cough clearance in patients with primary ciliary dyskinesia.[43]
We conclude that an ultrasonic nebulizer delivers a dose of amiloride to the airway surfaces of normal individuals that is sufficient to inhibit [Na.sup.+] transport for 3 to 5 h. Aerosol delivery of the drug results in a blood concentration 30 min afterwards, which appears to reflect drug delivered to the lung with early absorption across lung surfaces. The transbronchoscopic sampling technique is a useful method of assessing drug delivery to the airways.
ACKNOWLEDGMENTS: The authors thank Dr. K. Selinger, Glaxo Inc. Research Triangle Park, who measured amiloride concentrations in the airway surface liquid sample; Dr. John Gatzy, University of North Carolina, Chapel Hill, for helpful discussion; Joseph Robinson, Carla Foy, and Cynthia Taylor for technical assistance; and support from Glaxo Inc., Research Triangle Park, for the measurements of amiloride concentrations in plasma and urine samples.
REFERENCES
[1] Boucher RC. Human airway ion transport (part 1). Am J Respir Crit Care Med 1994; 150:271-81
[2] Boucher RC. Human airway ion transport (part 2). Am J Respir Crit Care Med 1994; 150:581-93
[3] Welsh MJ, Tsui L, Boat TF, et al. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, et al, eds. The metabolic and molecular bases of inherited diseases. 7th ed. New York: McGraw-Hill, 1966; 3799-876
[4] Davis PB, Drumm M, Konstan MW. Cystic fibrosis--state of the art. Am J Respir Crit Care Med 1996; 154:1229-56
[5] Knowles MR, Church NL, Waltner WE, et al. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med 1990; 322:1189-94
[6] Tomkiewicz RP, App EM, Zayas JG, et al. Amiloride inhalation therapy in cystic fibrosis: influence on ion content, hydration, and rheology of sputum. Am Rev Respir Dis 1993; 148: 1002-07
[7] Olivier KN, Bennett WD, Hohneker KW, et al. Acute safety and effects on mucociliary clearance of aerosolized uridine 5'-triphosphate +/- amiloride in normal adults. Am J Respir Crit Care Med 1996; 154:217-23
[8] Bennett WD, Olivier KN, Zeman KL, et al. Effect of uridine 5'-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am J Respir Crit Care Med 1996; 153:1796-1801
[9] Loffert DT, Ikle D, Nelson HS. A comparison of commercial jet nebulizers. Chest 1994; 106:1788-93
[10] Waldrep JC, Keyhani K, Black M, et al. Operating characteristics of 18 different continuous-flow jet nebulizers with beclomethasone dipropionate liposome aerosol. Chest 1994; 105:106-10
[11] Thomas SHL, O'Doherty MJ, Graham A, et al. Pulmonary deposition of nebulized amiloride in cystic fibrosis: comparison of two nebulisers. Thorax 1991; 46:717-21
[12] O'Doherty MJ, Thomas S, Page C, et al. Pulmonary deposition of nebulised pentamidine isethionate: effect of nebuliser type, dose and volume of fill. Thorax 1990; 45:460-64
[13] Mukhopadhyay S. When will nebulized chemotherapy come of age? Respir Med 1994; 88:245-47
[14] Knowles MR, Church NL, Waltner WE, et al. Amiloride in cystic fibrosis: safety, pharmacokinetics, and efficacy in the treatment of pulmonary disease. In: Cragoe EJ, Kleyman TR, Simchowitz L, eds. Amiloride and its analogs: unique cation transport inhibitors. New York. VCH Publishers, 1992; 301-16
[15] Hollie MC, Malone RA, Skofca RM, et al. Extreme variability in aerosol output of the DeVilbiss 646 Jet nebulizer. Chest 1991; 100:1339-40
[16] Jorres R, Nowak D, Rabe K, et al. Variability in aerosol output of the DeVilbiss 646 nebulizer [letter]. Chest 1992; 102:1636
[17] Selinger K. Measurement of amiloride in airway surface liquid utilizing HPLC and fluorescence detection. Biomed Chromatogr 1994; 8:219-23
[18] Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics 1982; 38:963-74
[19] Akaike H. An information criterion. Math Sci 1976; 14:5-9
[20] Smith JJ, Travis SM, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface liquid. Cell 1996; 85:229-36
[21] Bowler IM, Kelman B, Worthington D, et al. Nebulised amilonde in respiratory exacerbations of cystic fibrosis: a randomised controlled trial. Arch Dis Child 1995; 73:427-30
[22] Boucher RC, Stutts MJ, Knowles MR, et al. [Na.sup.+] transport in cystic fibrosis epithelia: abnormal rate and response to adenyl cyclase activation. J Clin Invest 1986; 78:1245-52
[23] Knowles M, Murray G, Shallal J, et al. Bioelectuc properties and ion flow across excised human bronchi. J Appl Physiol 1984; 56:868-77
[24] Boucher RC, Cotton CU, Gatzy JT, et al. Evidence for reduced [Cl.sup.-] permeability and increased [Na.sup.+] permeability in cystic fibrosis human primary cell cultures. J Physiol (Lond) 1988; 405:77-103
[25] Knowles MR, Gatzy JT, Boucher RC. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest 1983; 71:1410-17
[26] Anderson WA. Pharmacokinetics of amiloride by inhalation in adults, adolescents, and children [abstract]. Pediatr Pulmonol 1993; 9(suppl): 150-51
[27] Jones K, Liao E, Hohneker KH, et al. Pharmacokinetics of amiloride after inhalation and oral administration in adolescent and adult patients with cystic fibrosis [abstract]. Pediatr Pulmonol 1993; 9A(suppl):171-72
[28] Wood RE, Blair KC, Liao E, et al. Deposition of amiloride aerosol in the airways of children with CF, ages 4-11 years [abstract]. Pediatr Pulmonol 1992; 9A(suppl): Hot Science
[29] Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol 1992; 34:311-15
[30] Summers QA, Singh S, Honeywell RG, et al. The effects of respiratory manoevres and pharmacological agents on the pharmacokinetics of nedocromil sodium after inhalation. Br J Clin Pharmacol 1992; 33:431-38
[31] Hardy JG, Newman SP, Knoch M. Lung deposition from four nebulizers. Respir Med 1993; 87:461-65
[32] Mukhopadhyay S, Staddon GE, Eastman C, et al. The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Respir Med 1994; 88:203-11
[33] Ilowite JS, Gorvoy JD, Smaldone GC. Quantitative deposition of aerosolized gentamycin in cystic fibrosis. Am Rev Respir Dis 1987; 136:1445-49
[34] Weber A, Smith A, Williams-Warren J, et al. Nebulizer delivery of tobramycin to the lower respiratory tract. Pediatr Pulmonol 1994; 17:331-39
[35] O'Doherty MJ, Thomas S, Page C, et al. Differences in relative efficiency of nebulisers for pentamidine administration. Lancet 1988; 2:1283-86
[36] Hofmann T, Ziersch A, Senier I, et al. Benzamil and amiloride in CF nasal epithelium: time course of the effects on nasal potential difference in vivo [abstract]. Pediatr Pulmonol 1996; 13 (suppl):254
[37] Blank U, Hofmann T, Clauss W, et al. Different blocker kinetics of amiloride and phenamil on epithelial sodium conductances in cystic fibrosis nasal epithelium [abstract]. Pediatr Pulmonol 1996; 13 (suppl):255
[38] Rennard SI, Basset G, Lecossier D, et al. Estimation of the volume of epithelial lining fluid using urea as a marker of dilution. J Appl Physiol 1986; 60:532-38
[39] Von Wichert P, Joseph K, Muller B, et al. Bronchoalveolar ravage: quantitation of intraalveolar fluid? Am Rev Respir Dis 1993; 147:148-52
[40] Chinard FP. Quantitative assessment of epithelial lining fluid in the lung. Am J Physiol 1992; 263:L617-18
[41] Restrick LJ, Sampson AP, Piper PJ, et al. Inulin as a marker of dilution of bronchoalveolar ravage in asthmatics and normal subjects. Am J Respir Crit Care Med 1995; 151:1211-17
[42] App EM, King M, Helfesrieder R, et al. Acute and long term amiloride inhalation in cystic fibrosis lung disease: a rational approach to cystic fibrosis therapy. Am Rev Respir Dis 1990; 141:605-12
[43] Noone PG, Bennett WD, Zeman KL, et al. Cough clearance in patients with primary ciliary dyskinesia: effects of aerosolized uridine-5'-triphosphate and amiloride [abstract]. Am J Respir Crit Care Med 1995; 151:A464
(*) From the Cystic Fibrosis/Pulmonary Research and Treatment Center the Division of Pulmonary Medicine Department of Medicine (Drs. Noone Regnis and Knowles), the School of Pharmacy (Mr. Liu and Dr. Brouwer) Department of Biostatistics (Dr. Edwards) University of North Carolina at Chapel Hill, and the Royal Prince Alfred Hospital (Mr. Robinson) Sydney Australia Supported in part by grants from the National Institutes of Health (HL 42322 HL 42384, RR 00046), the Cystic Fibrosis Foundation (CFF L543) Omron Healthcare, Vernon Hills, Ill, the Winston Churchill Memorial Trust of Australia (J.A.R.) and Glaxo Wellcome Inc, for supporting measurements of amiloride concentrations
Manuscript received October 16 1996; revision accepted April 8 1997.
Reprint requests: Peadar G. Noone, MD, FCCP, Pulmonary Division, CB #7248, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-724S; email: pnoone@med.unc.edu.
COPYRIGHT 1997 American College of Chest Physicians
COPYRIGHT 2004 Gale Group