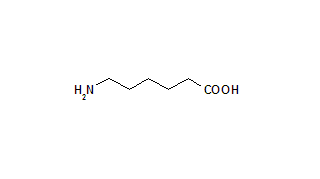
To determine the effects of prophylactic treatment with EACA for blood loss after cardipulmonary bypass surgery, 350 consecutive patients undergoing open-heart surgery were studied. One hundred seventy patients received an initial dose of 5 g of EACA prior to skin incision, followed by intravenous administration of 1 g/h for the next 6 to 8 h. The control group received saline solution in the same fashion. The EACA-treated group had decreased chest tube blood loss 24 h postoperatively. In addition, EACA-treated patients had fewer myocardial infarctions, cerebrovascular accidents or reoperations for bleeding. Treated patients needed fewer units of blood transfusions than the non-treated group. There was no incidence of hyperthrombotic state or other side effects in the EACA-treated group. We concluded that prophylactic treatment with EACA for open-heart surgery requiring extracorporeal circulation may reduce the total blood loss and the number of blood transfusions in a safe and tolerable manner.
(Chest 1989; 96:27-30)
Excessive bleeding is an alarming complication of open-heart surgery. There is no active source of bleeding in most cases, but generalized oozing is encountered. The decision to reoperate is sometimes controversial and difficult to make. Reexploration for excessive postoperative hemorrhage is commonly associated with increased incidence of morbidity and mortality.[1-3] Patients undergoing cardiac surgery with extracorporeal circulation are prone to severe coagulation disorders.[4-8] Hyperfibrinolysis, caused by an increased release of plasminogen activator which converts the plasminogen to plasmin,[6,7,9-13] is frequent in these patients.
The hemorrhage tendency that follows cardiopulmonary bypass is a complex process, initiated by trauma to the cellular components of the blood. Should activation of plasminogen develop or proceed, multiple coagulation defects may occur, including changes in antithrombotic titer, fibrinogen concentration, and a decrease in the number of circulating platelets. Early preventative treatment of hyperfibrinolysis decreases the breakdown of factor V and VIII. Presently, the correction of such defects may be accomplished only by administration of frozen plasma or fresh whole blood. Fibrinopeptides, released from the breakdown of fibrin, may interfere with the polymerization of fibrin and thus, act as anticoagulants. These anticoagulants are not susceptible to any presently known form of therapy. In an era whereby transfusions run a great risk of transmitting viral disease and adverse reactions, and concerns that demand for blood and blood products may exceed the supply, interest in pharmacologic methods to reduce the need for blood in cardiac surgery has increased.
Epsilonaminocaproic acid inhibits the proteolytic activity of plasmin[14,15] and the conversion of plasminogen to plasmin by plasminogen activator.[16,17] Epsilon aminocaproic acid, a synthetic antifibrinolytic agent, has been advocated by some investigators to control spontaneous hemorrhage after cardiac surgery with extracorporeal circulation.[18-22] Since excessive fibrinolysis is believed to represent a substantial factor in the hemorrhagic tendency that follows cardiopulmonary bypass, the efficacy of EACA as prophylactic therapy for control of blood loss in patients undergoing open-heart surgery with the aid of extracorporeal circulation, was investigated.
Clinical Materials and Methods
The research was conducted on patients undergoing open-heart surgery at Cooper Hospital/University Medical Center, Camden, NJ, and Deborah Heart and Lung Center, Browns Mills, NJ. Patients were randomly selected to receive either EACA or a placebo (normal saline solution) in a double-blind fashion. All patients signed a consent form for participation in the study, which was approved by the Institutional Review Board.
Included in the study were patients undergoing elective surgery for coronary artery disease (saphenous vein grafting), repair of myocardial aneurysms, valve replacement or combined procedures. No emergency procedures were included. All patients had preoperatively normal renal function.
A total of 350 consecutive patients were randomly assigned to one of two groups: 170 patients received an initial priming dose of 5 g of EACA (Amicar, Lederle Laboratories) prior to skin incision, followed by a continuous intravenous infusion of 1 gph over the next 6 to 8 h. One hundred eighty patients received saline solution in the same fashion. A standard anesthetic technique was used (intermittent positive-pressure ventilation, nitrous oxide, oxygen, narcotic analgesics and muscle relaxants). A membrane oxygenator (Bentley CM50, Bentley Laboratories Inc, Irvine, CA) was employed in all cases. Multidose crystalloid cardioplegia and systemic hypothermia (25[degrees]C) were instituted in all cases. Heparin was administered at an initial dose of 300 units/kg and the activated clotting time was maintained at greater than 400 s during cardiopulmonary bypass. A 2.5-L asanguineous solution was used to prime the pump circuit. Nonpulsatile flow (2.0 to 2.4 L/min/[m.sup.2]) and mean arterial pressure between 60 and 85 mm Hg were maintained during cardiopulmonary bypass with phenylephrine or sodium nitroprusside as required.
Residual heparinization was reversed with protamine sulfate (1 mg/kg). Half of the dose was administered as bolus and half as a continuous infusion. Coagulation profiles (activated clotting time, prothrombin time, thromboplastin time, platelet and complete blood cell counts, fibrin split products, fibrinogen level) were assessed preoperatively, immediately after resolution of cardiopulmonary bypass and while in the Intensive Care Unit. Patients with preoperative coagulation disorders were excluded from the study. Patients had pericardial and mediastinal drains inserted and continuous low-grade suction instituted. The volumes of blood that had been adminstered and the amount of blood lost into the drainage system were recorded 24 h postoperatively. The postoperative blood loss through the drainage tubes was measured in cubic centimeters per 24 h. In the majority of cases, drainage tubes were removed at or 24 h after the operation. Crystalloid and colloid solutions were administered postoperatively to maintain left atrial pressure between 8 and 10 mm Hg. Packed red blood cells were transfused if the hemoglobin level fell to less than 100 g/L. Patients also were monitored for postoperative complications. Other preoperative, intraoperative and postoperative variables were recorded during the study. Only relevant data have been presented here. Statistical evaluation of the results was done using paired-sample t-test. Results are presented as mean [+ or -] standard deviation. A p value of less than 0.05 was considered statistically significant.
Results
Data were analyzed from the two patient groups, who were divided according to the prophylactic administration of EACA or placebo. Table 1 represents the demographic and clinical data for the two patient groups. There were no statistical differences in age, sex, diagnostic and operative procedures as well as preoperative and postoperative coagulation profiles (data not shown). However, there is a statistically significant reduction in blood loss (cubic centimeters per 24 h) in the EACA group (617.2 [+ or -] 43.9 vs 883.2 [+ or -] 29.3, p<0.05). Six patients in the control group required reoperation for excessive hemorrhage. In all six cases no bleeding site was identified, but generalized oozing was encountered. None of the patients receiving prophylactic EACA required reoperation for bleeding. Ten patients (5.5 percent) in the control group had a documented intraoperative myocardial infarction. This was observed in only four patients (2.3 percent) receiving EACA. One patient in the control group had an early postoperative cerebrovascular [TABULAR DATA OMITTED] accident. This type of complication was not seen in the study group. The incidence of death was equal in both groups and not related to the postoperative bleeding. There was no incidence of adverse reaction (gastrointestinal, myopathy, convulsions, hypotension or hyperkalemia) as a result of the administration of EACA. We found no incidence to support any concerns regarding the possible existence of a thrombotic state. The amount of blood transfusions required was strikingly different. Patients treated with EACA received 2.8 [+ or -] 2.0 units of blood vs 4.2 [+ or -] 2.3 units for the untreated group, representing a statistically significant difference (p<0.05).
Discussion
Annually, greater than 200,000 open-heart operations are performed throughout the United States.[23] Cardiac surgical patients usually require more than 4 units of donor blood postoperatively.[24] These cases consume over 25 percent of all blood used.[25] Patients who receive blood are exposed to an increased risk of transmitted viral diseases and adverse reactions. Excessive transfusions contribute to acid-base disturbances, dilutional coagulopathies, unstable hemodynamics, and potential for subsequent pulmonary problems. Even more important, most blood banks are presently facing severe shortages and an imbalance between the blood supply and demand.
Improvements in cardiac operative techniques and extracorporeal circulation have paralleled reduction in postoperative hemorrhage. However, excessive blood loss still remains one of the potential sources of morbidity and mortality.
Hemorrhagic diathesis after cardiopulmonary bypass has been ascribed in part to hyperfibrinolysis due to increased plasminogen activator activity. This appears after sternotomy, reaches maximal intensity during and immediately upon termination of cardiopulmonary bypass[20,26] and persists 1 to 2 h postoperatively.[19] Some investigators have demonstrated that epicardial and mesothelial surfaces (eg, pleura, pericardium and peritoneum) contain fibrinolytic activator activity.[26] Plasmin exerts its proteolytic effect on several plasma proteins, including the coagulation components of fibrinogen, factor V and factor VIII. Lowering the concentration of these blood coagulation factors in plasma, combined with the inhibitory effect of some fibrinogen degradation products on platelet aggregation and thrombin activity, induces an undesired and potentially hazardous hemorrhage defect. In these cases, neutralization of the hyperfibrinolytic state with inhibitors of plasmin activity is required.
Epsilon aminocaproic acid, a synthetic mono-aminocarboxylic acid with a structure closely related to lysine, has been advocated to be effective in reducing blood loss in a variety of clinical conditions.[26-31] It appears that EACA interacts with fibrin, protecting it against protolysis. This may be responsible for both its protective activity against a spectrum of proteolytic enzymes and its anticoagulant effect. It was shown that EACA prolongs partial thromboplastin time and one-stage prothrombin time.[19] The primary role of EACA is to effectively saturate the lysin-binding sites of plasminogen, the key substance of the fibrinolytic system. This action prevents the dissolution of clots by inhibiting plasminogen activators. Epsilon aminocaproic acid also has a direct antiplasmin activity which inhibits its release. An increased beta-glucuronidase level, reflecting lysosomal release from cells, often is found following extracorporeal circulation. This may suggest increased cellular damage, with changes in membrane permeability, cellular disruption or both. The rise in beta-glucuronidase is blocked by EACA administration.[32]
Epsilon aminocaproic acid was utilized as an antifibrinolytic agent in an earlier study to reduce blood loss after cardiopulmonary bypass, based on the rationale that fibrinolysis is an important determinant factor in the postoperative hemorrhage diathesis. However, published results are inconclusive and very controversial.[18-22,31,33-35] The indication for therapeutic and/or prophylactic use of EACA also remains controversial.[9,21,36] Some investigators manifest accentuated precautions in administering the drug due to presumed side effects and the possibility of producing a hypercoagulability state.[33-35,37]
Several studies have demonstrated that EACA does not increase the incidence of thrombotic episodes.[21,22] Nevertheless, there are fears expressed among clinicians that EACA treatment could contribute to systemic thrombosis.[32,33,36,38]
This double-blind prospective study was designed to test the safety and efficacy of EACA as prophylatic treatment for postperfusion bleeding after open-heart surgery. The results of this study have shown statistically significant reductions in blood loss when EACA is used prophylactically. Treatment with EACA was safe and tolerable in this clinical setting. We did not observe any circumstances where a hypercoagulability state appeared to be present. We do not know if the differences in incidence of myocardial infarction and/or cardiovascular accidents may be attributed to differences in treatment between the two groups. We observed a statistically significant reduction of blood transfusions after prophylactic use of EACA. This finding may have major implications in the actual hospital setting where blood bank resources are limited. Our results coincide with other published papers;[22] however, this study extends the use of EACA to a variety of pathologic entities necessitating open-heart surgery with increasing risk of bleeding.
In conclusion, this study tries to answer a controversial issue: whether prophylactic use of EACA may prevent blood loss after cardiopulmonary bypass. We believe that if the activation of plasminogen is allowed to proceed, multiple coagulation defects may develop which are not responsive to treatment. An overwhelming imbalance toward fibrinolysis is complicated by an increased level of fibrin degradation products, which affects many steps of the coagulation cascade. Therefore, the sooner EACA is administered (ie, prophylactically), the less chance there is for hemorrhage to occur. The results of our study defend this rationale and have clearly shown that routine use of EACA prophylactically may have its clinical usefulness.
ACKNOWLEDGEMENTS: The authors thank Mrs. Lorraine Robinson and Ms. Karen Finn for their assistance in preparing this manuscript.
References
[1] Talamonti MS, LoCicero J III, Hoyne WP, Sanders JH, Michaelis LL. Early re-exploration for excessive post-operative bleeding lowers wound complication rates in open heart surgery. Am Surg 1987; 53:102-04
[2] Sanfelippo PM, Danielson GK. Complications associated with median sternotomy. J Thorac Cardiovase Surg 1972; 63:419-23
[3] Serry C, Bleck PC, Javid H, Hunter JA. Sternal wound complications. J Thorac Cardiovasc Surg 1980; 80:861-67
[4] Beall AC Jr, Strawn JR, Tillerty WV, Cooley DA. Effects of extracorporeal circulation on fibrinolytic activity in man. Clin Res 1966; 14:238
[5] Gans H, Lillehei CW, Krivit W. Problems in hemostasis during open-heart surgery: I. on the release of plasminogen activator. Ann Surg 1961; 154:915
[6] Gans H, Krivit W. Problems in hemostasis during open-heart surgery: III. epsilon-aminocaproicaid as an inhibitor of plasminogen activitor. Ann Surg 1962; 155:268.
[7] Gibbon JA Jr, Camishion R. Problems in hemostasis with extracorporeal bypass. Ann NY Acad Sci 1964; 115:195
[8] Bachmann F, McKenna R, Cole ER, Najafi H. The hemostatic mechanism after open-heart surgery: I. studies on plasma coagulation factors and fibrinolysis in 512 patients after extracorporeal circulation. J Thorac Cardiovasc Surg 1975; 70:76-85
[9] Gomes MM, McGoon DC. Bleeding patterns after open-heart surgery. J Thorac Cardiovasc Surg 1970; 60:87-97
[10] Eckert H, Montgomery D, Aberdeen F. Fibrinolysis during extra-corporeal circulation: comparison of the effects of disc and membrane oxygenators. Circ Res 1971; 28:512-17
[11] Gralnick HR, Fischer RD. The hemostatic response to open heart operations. J Thorac Cardiovasc Surg 1971; 61:909-15
[12] Mori F, Nakahara Y, Kurata S, Furukawa S, Esato KF, Mohri M. Late changes in hemostatic parameters following open heart surgery. J Cardiovasc Surg 1982; 23:458-62
[13] Porter JM, Silver D, Durham NC. Alterations in fibrinolysis and coagulation associated with cardiopulmonary bypass. J Thorac Cardiovasc Surg 1968; 56:869-78
[14] Guileman P. L'activite antifibrinolytique de l'acide epsilon amino-caproique: ses applications therapeutiques. Rev Prat (Paris) 1966; 16:769-84
[15] Brockway WJ, Castellino FJ. The mechanism of the inhibition of plasmin activity by [epsilon]-aminocaproic acid. J Biol Chem 1971; 246:4641-47
[16] Kaplan AP, Austen KF. The fibrinolytic pathway of human plasma: isolation and characterization of the plasminogen pro-activator. J Exp Med 1972; 136:1378-93
[17] Soter NA, Austen KF, Gigli I. Inhibition by [epsilon]-aminocaproic acid of the activation of the first component of the complement system. J Immunol 1975; 114:928-32
[18] Midel AI, Grady LH, Bloodwell RD, Beall AC Jr, Yashar JJ, Cooley DA. Epsilon aminocaproic acid for bleeding after cardiopulmonary bypass. Ann Thorac Surg 1971; 11:577
[19] Ambrus JL, Ambrus CM, Stutzman L, Stutzman L, Shimert G, Niswander KR, et al. Treatment of fibrinolytic hemorrhage with proteinase inhibitors: a preliminary report. Ann NY Acad Sci 1988; 146:625-41
[20] McClure PD, Izsak J. The use of epsilon-aminocaproic acid to reduce bleeding during cardiac bypass in children with congential heart disease. Anesthes 1974; 40:604-08
[21] Lambert CJ, Marengo-Row AJ, Leveson JE, Green RH, Thiele JP, Geisler GF, et al. The treatment of postperfusion bleeding using epsilon-aminocaprioc acid, cryoprecipitate, fresh frozen plasma, and protamine sulfate. Ann Thorac Surg 1979; 28:440-44
[22] Salm TJF, Ansell JE, Okike ON, Marsicano TH, Lew R, Stephenson WP, et al. The role of epsilon-aminocaproic acid in reducing bleeding after cardiac operation: a double-blind randomized study. J Thorac Cardiovasc Surg 1988; 95:538-40
[23] Kennedy RH, McGoon DC, Smith HC, Kurland LI. Trends in cardiac surgery in the United States. N Engl J Med 1985; 312:119-20 [24] Dodsworth H, Dudley HAF. Increased efficiency of transfusion practice in routine surgery using pre-operative antibody screening and selective ordering with an abbreviated cross-match. Br J Surg 1985; 72:102-04
[25] Freedman J, Lim C, Wright J. Changing patterns of transfusion practice in a tertiary area hospital from 1977-1984. Can Anaesth Soc J 1986; 33:458-65
[26] Rhodes GR, Silver D. Periepicardial fibrinolytic activity: relation to cardiac bleeding. Surg 1975; 78:230-37
[27] Smith RB, Riach P, Kaufman JJ. Epsilon aminocaproic acid and the control of post-prostatectomy bleeding: a prospective double-blind study. J Urol 1984; 131:1093-95
[28] Salcedo JR, Silverstein CE. Post-biopsy bleeding in renal allograft: successful treatment with epsilon aminocaproic acid (Letter). J Urol 1982; 127:783
[29] Kang Y, Lewis JH, Navalgund A, Russel MW, Bontempo FA, Niren LS, et al. Epsilon-aminocaproic acid for treatment of fibrinolysis during liver transplantation. Anaesthesia 1987; 66:766-73
[30] Kasper CK, Dietric SL. Comprehensive management of haemophilia. Clin Haemat 1985; 14:489-512
[31] Miller RD, Brzica SM Jr. Blood, blood components, colloids and autotransfusion therapy. In: Miller RD, ed. Anaesthesia. New York: Churchill-Livingstone, 1986; 1329-67
[32] Tice DA, Worth MJ Jr. Recognition and treatment of postoperative bleeding associated with open-heart surgery. Ann NY Acad Sci 1966; 146:745-53
[33] Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs 1985; 29:236-61
[34] Saussine M, Delpech S, Allien M, Grolleau D, Daures MF, Coulon P, et al. Saignement apres circulation extracorporelle et acide epsilon amino-caproique. Ann Fr Anestha Reanim 1985; 4:403-05
[35] Anderson L, Nilsoon IM, Collen S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann NY Acad Sci 1968; 146:642-58
[36] Garcia JB, Pakrashi BC, Mary DA, Tandon RK, Ionescu MI. Post-operative blood loss after extracorporeal circulation for heart valve surgery. J Thorac Cardiovasc Surg 1973; 65:487-96
[37] Gralnick HR, Fischer RD. The hemostatic response to open-heart operations. J Thorac Cardiovasc Surg 1971; 61:909-15
[38] Gralnick HR, Greipp P. Thrombosis with epsilon aminocaproic acid therapy. AJCP 1971; 56:151-54
COPYRIGHT 1989 American College of Chest Physicians
COPYRIGHT 2004 Gale Group