Extracorporeal membrane oxygenation (ECMO) was developed as a supportive therapy for severe respiratory failure. It has been shown to be life-saving in neonates and children with isolated respiratory failure, however, its usefulness in adults remains controversial. We report the successful use of ECMO in an adult patient with severe hypoxemic respiratory failure secondary to diffuse alveolar hemorrhage from Wegener granulomatosis.
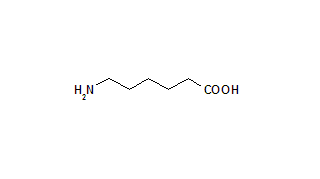
Key words: aminocaproic acid; antineutrophil cytoplasmic antibody; diffuse alveolar hemorrhage; extracorporeal membrane oxygenation; respiratory failure; vasculitis; Wegener's granulomatosis
Abbreviations: ANCA = antineutrophil cytoplasmic antibody; DAH = diffuse alveolar hemorrhage; ECMO = extracorporeal membrane oxygenation; FI[O.sub.2] = fraction of inspired oxygen; MUSC = Medical University of South Carolina; PEEP = positive end-expiratory pressure; WG = Wegener granulomatosis
**********
Wegener granulomatosis (WG) is characterized by the coexistence of small-vessel vasculitis and granulomas in multiple organ systems. Classically, WG involves the kidneys, and the upper and lower respiratory tracts.
For editorial comment see page 9
Although many patients with WG have capillaritis seen on surgical lung biopsy specimens, diffuse alveolar hemorrhage (DAH) is a rare complication that carries anextremely high fatality rate. Support of the respiratory and renal systems is often required in conjunction with aggressive therapy for the underlying vasculitis. Extracorporeal membrane oxygenation (ECMO) has been shown to be life-saving for a select population of adults with respiratory failure, although the presence of systemic disease is generally considered to he a contraindication. We report the sixth adult patient with DAH from antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis that was successfully supported with ECMO. (1-3)
CASE REPORT
A 26-year-old white woman with newly diagnosed WG was admitted to the Medical University of Sooth Carolina (MUSC) for malaise, fatigue, left-sided flank pain, and hemoptysis of 1-week duration. The patient reported having symptoms of chronic sinusitis, migratory, joint pain, hearing loss, and decreased appetite for months. One month prior to hospital admission, she was found to have an ANCA with a cytoplasmic pattern of 1:320. A previous maxillary sinus biopsy had revealed extensive sinusitis with focal multinucleated giant cells. Her family history was significant for a maternal grandmother with WG.
A physical examination revealed a temperature of 96.4[degrees]F, a pulse 91 beats/min, a respiratory rate of 24 breaths/min, and a BP of 113/78 mm Hg. She had pale conjunctiva, a right myringotomy tube, bilateral crackles on auscultation, aid a left knee effusion. Her chest radiograph (Fig 1, top) and CT scan (Fig 1, bottom) revealed bilateral alveolar opacities and a left upper lobe cavitary lesion. Laboratory data were significant for hematuria (72 RBCs per high-power field), a creatinine level of 3.9 mg/dL, a BUN of 24 mg/dL, a WBC count of 15,900 cells/[micro]L, a hemoglobin count of 9.1 g/dL, hematocrit of 23.5%, and a platelet count of 509,000 cells/[micro]L.
[FIGURE 1 OMITTED]
The patient was started on therapy with IV cyclophosphamide, 150 mg once daily, and dexamethasone, 60 mg three times per day. On hospital day 3, the patient was transferred to the medical ICU for increasing hemoptysis and hypoxemia. A few hours after transfer, the patient was emergently intubated secondary to worsening hypoxemia and increased work of breathing. She had massive hemoptysis from the endotracheal tube and required aggressive manual bagging to maintain oxygen saturation at >85%. As a result, she developed hemodynamic compromise from bilateral tension pneumothoraces requiring the emergent placement of bilateral chest tubes. Oxygen saturations continued to decline despite a fraction of inspired oxygen (FI[O.sub.2]) of 1.0, multiple modes of ventilation (including high-frequency oscillatory ventilation), various levels of positive end-expiratory pressure (PEEP), sedation, and paralysis.
Within 4 h of intubation, the decision was made to initiate venovenous ECMO. The patient was taken to the operating room for placement of a 20F cannula in her right internal jugular vein and a 23F cannula in her right femoral vein. We drained blond from the patient's femoral vein and returned blood through her internal jugular vein in order to avoid recirculation. Following the initiation of ECMO, tidal volumes were decreased and FI[O.sub.2] was lowered in order to prevent barotrauma and oxygen toxicity. The patient's initial ventilator settings were pressure-regulated volume control, a rate of 12 breaths/min, a tidal volume of 350 mL (5 mL/kg), a PEEP of 12 mm Hg, and an FI[O.sub.2] of 0.5. Due to peak inspiratory pressures of > 30 mm Hg, her tidal volume was further reduced to 325 mL, and PEEP was reduced to 10 mm Hg. The initial ECMO settings were a flow of 3.5 L/min and a sweep of 2 L/min. Because of the risk of continued alveolar hemorrhage, anticoagulation therapy was avoided for the first 48 h by using a centripetal pump, which does not require systemic heparinization. She was subsequently changed to a traditional roller pump, and she received full anticoagulation. During her ECMO run, flows were maintained between 2 and 3 L/min. and the sweep was gradually increased to 4.5 L/min. Beginning with hospital day 9, we began attempting breathing trials without ECMO. Over the next few days, no major changes were made with the ventilator, and the chest radiograph findings gradually improved. On hospital day 16 (ECMO day 12), the patient was able to remain without ECMO for our goal of 4 h with oxygen saturations in the mid-90% range. At the conclusion of this trial, an arterial blood gas analysis revealed a pH of 7.27, a PC[O.sub.2] of 50 mm Hg, and a P[O.sub.2] of 80 mm Hg. The patient was subsequently decannulated at the bedside.
Infection was excluded, and DAH was confirmed by BAL, which was performed shortly after the initiation of ECMO. Her WG was aggressively treated with high-dose cyclophosphamide (300 mg IV daily) and dexamethasone (60 mg IV three times per day). She underwent plasmapharesis for 5 days followed by therapy with IV Ig (490 mg/kg/d) for an additional 5 days. Because the rise of ECMO and aggressive immunosuppression interfered with our ability to monitor for signs and symptoms of infection, the patient received empiric therapy with broad-spectrum antibiotics, and daily surveillance cultures were collected. She also underwent hemodialysis followed by continuous venovenous hemofiltration through the ECMO circuit.
Her hospital course was complicated by profuse bleeding from an additional chest tube that had been placed emergently for a recurrent right-sided tension pneumothorax. Despite decreasing her activated clotting time to 140 to 160 s, the transfusion of platelets to > 125,000 cells/[micro]L, and transfusion with fresh-frozen plasma, she continued to bleed. Therefore, therapy with high-dose aminocaproic acid (30 mg/kg/h) was started. Two days after the initiation of therapy with aminocaproic acid, the patient developed an anion gap metabolic acidosis with an associated osmolar gap. Other potential causes of acidosis were ruled out, and the acidosis resolved with a reduction in the dose of aminocaproic acid to 10 mg/kg/h.
A thoracotomy for the evacuation of a right hemothorax and decortication was performed, allowing successful extubation on day 29. Figure 2 illustrates a schematic representation of the major events in the ICU. The patient continued to gradually improve and was discharged home on hospital day 58 with normal pulmonary and renal function.
[FIGURE 2 OMITTED]
DISCUSSION
ECMO was developed as a supportive therapy for severe respiratory failure, and has been shown to be life-saving in neonates and children with respiratory failure. (4-5) When conventional mechanical ventilation fails, ECMO can support oxygenation and ventilation, thus enabling the ventilator settings to be markedly reduced. This can help to prevent further lung damage cruised by high airway pressures and oxygen toxicity, while allowing more time for aggressive treatment of the underlying pathology.
The usefulness of ECMO in adults remains controversial. An earlier study concluded that the use of ECMO in adult patients with ARDS did not significantly change mortality. (6) However, this study is frequently- criticized because of high mortality rates in patients enrolled in both arms of the study, the lack of ECMO experience by the majority of participating centers, and the continued use of high ventilator settings while patients were receiving ECMO. ECMO has been shown to be beneficial for a selective population of adults with respiratory failure. (7) Table 1 shows the guidelines used at MUSC for the initiation of ECMO in adults who meet ARDS criteria. (8)
Kolovos et al (9) reported a case series of eight pediatric patients who were successfully treated with ECMO after developing respiratory failure secondary to DAH. Although the presence of systemic disease is generally considered to be a contraindication to ECMO, two patients had WG and two had systemic lupus erythematosus. This therapy also has been used successfully in five adult patients with DAH secondary to ANCA-associated vasculitis. (1-3)
DAH is one of the most serious complications of ANCA-associated vasculitis. The morality rate exceeds 80% when the Pa[O.sub.2]/FI[O.sub.2] ratio falls to < 100 mm Hg in mechanically ventilated patients. Although immunosuppressive therapy with glucocorticoids and cyclophosphamide have dramatically improved survival, (10) supportive therapies for complications affecting vital organs is often necessary. The use of IV Ig and plasmapheresis also has shown some benefit in select cases of ANCA-associated vasculitis. (11,12) After conventional methods of mechanical ventilation failed, we used ECMO to support the pulmonary system. By allowing for a significant decrease in ventilator settings, ECMO may have had a direct beneficial effect in improving alveolar hemorrhage.
This case is also illustrative of one of the potentially serious complications of ECMO. Heparinization of the patient is required to reduce the risk of clotting in the traditional roller ECMO circuit but results in an increased risk of bleeding, We encountered this complication despite maintaining a lower therapeutic range of anticoagulation to ensure adequate ECMO flow rates while attempting to minimize this risk. Aminocaproic acid interferes with fibrinolysis through the inhibition of plasminogen activators and may reduce the risk of bleeding complications in patients fully heparinized for ECMO. (13) To our knowledge, anion gap metabolic acidosis has not been reported with the use of aminocaproic acid.
Although, ECMO is a highly invasive procedure with a significant risk of bleeding complications, it may be lifesaving in acute respiratory failure that is refractory to conventional mechanical ventilation. It is especially useful if used early in the hospital course of patients with potentially reversible pathology and without underlying chronic lung disease. We report the sixth adult patient with DAH from ANCA-associated vasculitis that was successfully supported with ECMO. In conclusion, ECMO should be considered for supportive therapy in patients with DAH from ANCA-associated vasculitis when conventional mechanical ventilation has failed.
* From the Department of Medicine (Dr. Ahmed), College of Medicine (Mr. Aziz), Division of Emergency and Critical Care (Dr. Coehran), Department of Pediatrics, and Division of Pulmonary, Critical Care, Allergy, and Clinical Immunology (Dr. Highland), Medical University of South Carolina, Charleston, SC.
Manuscript received November 25, 2003; revision accepted February 12, 2004.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: Kristin Highland, MD, FCCP, Assistant Professor of Medicine, Division of Pulmonary, Critical Care, Allergy, and Clinical Immunology, 96 Jonathan Lucas St, Suite 812 CSB, PO Box 2,50623, Charleston, SC 2942,5; e-mail: highlakb@musc.edu.
REFERENCES
(1) Hartmann A, Nordal KP, Svennevig J, et al. Successful use of artificial lung (ECMO) and kidney in the treatment of a 20-year-old female with Wegener's syndrome. Nephrol Dial Transplant 1994; 9:316-319
(2) Rosengarten A, Elmore P, Epstein J. Long distance road transport of a patient with Wegener's Granulomatosis and respiratory failure using extracorporeal membrane oxygenation. Emerg Med (Fremantle) 2002; 14:181-187
(3) Loscar M, Hummel T, Haller M, et al. ARDS and Wegener's granulomatosis. Anaesthesist 1997; 46:969-973
(4) UK Collaborative ECMO Trial Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet 1996; 348:75-82
(5) O'Rourke PP, Stolar CJ, Zwischenberger JB, et al. Extracorporeal membrane oxygenation: support for overwhelming pulmonary failure in the pediatric population; collective experience from the extracorporeal life support organization. J Pediatr Surg 1993; 28:523-528
(6) Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA 1979; 242:2193-2196
(7) Peek GJ, Moore HM, Moore N, et al. Extracorporeal membrane oxygenation for adult respiratory failure. Chest 1997; 112:759-764
(8) Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967; 2:319-323
(9) Kolovos NS, Schuerer DJ, Moler FW, et al. Extracorporeal life support for pulmonary hemorrhage in children: a ease series. Crit Care Med 2002; 30:577-580
(10) Langford CA, Talar-Williams C, Barron KS, et al. A staged approach to the treatment of Wegener's granulomatosis: induction of remission with glucocorticoids and daily cyclophosphamide switching to methotrexate for remission maintenance. Arthritis Rheum 1999; 42:2666-2673
(11) Jayne DR, Chapel H, Adu D, et al. Intravenous immunoglobulin for ANCA-associated systemic vasculitis with persistent disease activity. QJM 2000; 93:433-439
(12) Regan MJ, Hellmann DB, Stone JH. Treatment of Wegener's granulomatosis. Rheum Dis Clin North Am 2001; 27:863-886
(13) Wilson JM, Bower LK, Fackler JC, et al. Aminocaproic acid decreases the incidence of intracranil hemorrhage and other hemorrhagic complications of ECMO. J Pediatr Surg 1993; 28:536-540
COPYRIGHT 2004 American College of Chest Physicians
COPYRIGHT 2004 Gale Group