OraPharma, Inc., a pharmaceutical company
specializing in the dental market, is launching a periodontal disease
patient education and awareness campaign designed to encourage patients to
inquire about gum disease and periodontal health, while also encouraging
them to choose the comprehensive treatment of periodontal disease if it is
diagnosed.
The campaign is based on recently conducted dental consumer market
research* that came to four main conclusions:
The research also showed that when patients were educated, they would be
more willing to follow their dental practitioners' recommendations for
treatment and understood the need for adjunct antibiotic therapies to help
reduce the bacteria left behind by scaling and root planing.
According to Michael Cavanaugh, OraPharma's Executive Director of
Marketing, "The survey validates what we've always suspected -- the primary
reason patients delay treatment of periodontal disease is due to a lack of
understanding of the true nature of periodontal disease -- while it may be
painless and go unnoticed by the patient for years -- it's a chronic
infection that needs to be treated like any other infection in the body."
The OraPharma Periodontal Disease Patient Education Campaign consists of
several colorful
in-office patient education tools including brochures, patient self-exam
cards and other related pieces that are available to dental professionals
through the OraPharma account management and customer service teams.
These unbranded tools provide patients with detailed information regarding
periodontal disease, risk factors and treatment options embedded within
market research-validated visuals designed to catch and hold the attention
of dental patients and direct them to talk to their dental professional for
more information.
The in-office pieces are designed for the busy dental professional to
quickly educate and inform a patient about their periodontal infection
while also guiding them through the treatment options.
According to Dr. Sheri B. Doniger, a dental industry author and practicing
dentist in Lincolnwood, Illinois, "OraPharma's new education materials make
it easy for dental professionals to explain to their patients that
periodontal disease is a chronic oral infection. This campaign will be
extremely useful in increasing patients' knowledge of periodontal disease,
making the dental professionals' job of diagnosing and treating this
prevalent disease much easier."
About OraPharma, Inc.
OraPharma, Inc., is a specialty pharmaceutical company that develops
and commercializes therapeutics for oral health. OraPharma is dedicated to
the dental community, specifically the periodontal space with its lead
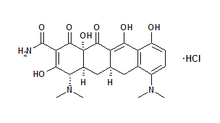
product, ARESTIN® (minocycline hydrochloride) Microspheres, 1 mg.
In addition to ARESTIN®, OraPharma also distributes Oraqix®
(lidocaine and prilocaine periodontal gel) 2.5% / 2.5%, a new sub-gingival
local anesthetic periodontal gel, indicated for adults who require
localized anesthesia in periodontal pockets during scaling and/or root
planing and is manufactured by Dentsply Pharmaceuticals.
Along with ARESTIN® and Oraqix®, OraPharma also promotes REACH®
ACCESSTM Daily Flosser, REACH® CLEAN BURST TM Dental Floss and ACT®
Anticavity Fluoride Rinse, distributed by McNeil-PPC, Inc., to the dental
community. For more information, visit www.orapharma.com
About ARESTIN®
OraPharma's flagship product ARESTIN® (minocycline hydrochloride)
Microspheres, 1 mg., is indicated as an adjunct to scaling and/or root
planing procedures for reduction of pocket depth in patients with adult
periodontitis. ARESTIN® may be used as part of a periodontal maintenance
program which includes good oral hygiene and scaling and root planing.
ARESTIN® contains minocycline, a tetracycline derivative, and therefore
should not be used in children and in pregnant or nursing women. The use
of drugs of the tetracycline class during tooth development may cause
permanent discoloration of the teeth.
The most common treatment-emergent adverse events were headache (9.0%),
infection (7.6%), flu syndrome (5.0%), and pain (4.3%). These occurred at
a similar rate to scaling and/or root planing and scaling and/or root
planing + placebo. For more information, visit www.arestin.com.
* Data on file, OraPharma, Inc. patient research 2004
Contact:
Michael Ventriello
Email Contact
www.lanmarkgroup.com