OraPharma, Inc., a pharmaceutical company
specializing in products for the dental market, was rated as the top
company for customer support by a survey of dental health professionals
recently conducted by Health Products Research, Inc.® (HPR).
According to James Charnetski, Vice President of Market Research/Health
Products Research, "The fact that over three-quarters of the dental offices
surveyed cited OraPharma as providing the best level of support to their
practice out of companies with an interest in the management of periodontal
disease clearly places the organization as the best in its class. A score
of 50% is considered a significant accomplishment -- the 76% level
demonstrates OraPharma's commitment to providing unsurpassed service and
support for its customers."
The extensive blinded research findings were the result of HPR's
Metropolitan Area Promotional Audit (MPA). This self-reported diary was
completed by 647 randomly selected offices from across the U.S. Offices
were queried on their hygiene and periodontal disease treatment protocols
as well as the products they use and the companies that support those
products.
"We are very proud of the HPR survey result," said Michael Cavanaugh,
Executive Director, Marketing. "Our commercial team received very high
ratings from dental practitioners, especially pertaining to our account
managers' professionalism and our innovative support of dental
professionals and their patients."
About OraPharma, Inc.
OraPharma, Inc., is a specialty pharmaceutical company, which discovers,
develops, and commercializes therapeutics for oral health. OraPharma is
dedicated to the dental community, specifically the periodontal space with
its lead product, ARESTIN® (minocycline hydrochloride) Microspheres, 1
mg.
In addition to ARESTIN®, OraPharma also distributes Oraqix® (lidocaine
and prilocaine periodontal gel) 2.5% / 2.5%, a new sub-gingival local
anesthetic periodontal gel, indicated for adults who require localized
anesthesia in periodontal pockets during scaling and/or root planing and is
manufactured by Dentsply Pharmaceuticals.
Along with ARESTIN® and Oraqix®, OraPharma also promotes REACH®
ACCESS(TM) Daily Flosser, REACH® CLEAN BURST(TM) Dental Floss and ACT®
Anticavity Fluoride Rinse, distributed by McNeil-PPC, Inc., to the dental
community.
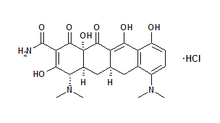
About ARESTIN®
OraPharma's flagship product ARESTIN® is indicated as an adjunct to
scaling and/or root planing procedures for reduction of pocket depth in
patients with adult periodontitis. ARESTIN® may be used as part of a
periodontal maintenance program which includes good oral hygiene and
scaling and/or root planing.
ARESTIN® contains minocycline, a tetracycline derivative, and therefore
should not be used in children and in pregnant or nursing women. The use
of drugs of the tetracycline class during tooth development may cause
permanent discoloration of the teeth.
The most common treatment-emergent adverse events were headache (9.0%),
infection (7.6%), flu syndrome (5.0%), and pain (4.3%). These occurred at
a similar rate to scaling and/or root planing and scaling and/or root
planing + placebo.
About Oraqix®
Oraqix,® manufactured for OraPharma, Inc. by Dentsply Pharmaceuticals, is
the first FDA-approved subgingival anesthetic indicated for use in adults
requiring anesthesia for scaling and/or root planing procedures. The
patient friendly, needle-free application avoids patients concerns about
needles and injections. Oraqix® is a trademark of Dentsply
Pharmaceuticals.
For more information, visit www.orapharma.com or call 1-866-273-7846.
Contact:
Lanmark Group
Michael Ventriello
mventriello@lanmarkgroup.com
www.lanmarkgroup.com