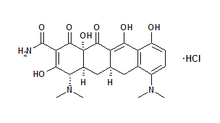
The U.S. Food and Drug Administration has approved minocycline (Arestin) microspheres for the adjunctive treatment of adult periodontitis following scaling and root planing. Periodontal disease is a vastly undertreated health problem that carries serious health risks. An estimated 50 million persons in the United States have periodontal disease. Treatment is important because the disease may be linked to conditions such as coronary heart disease, stroke, diabetes and low-birth-weight preterm infants.
According to the drug manufacturer, periodontal disease destroys supporting gum tissue and bone, forming "pockets" around the teeth in which plaque and bacteria accumulate. The severity of periodontal disease is measured in part by the depth of these pockets. In a healthy state, the depth of unattached gums is 2 to 3 mm. Patients with pockets with a depth of more than 4 mm have periodontal disease and patients with pocket depth of more than 6 mm have advanced periodontal disease.
Through microsphere technology, minocycline is delivered beneath the gum, directly into the infected periodontal pocket after deep cleaning of the teeth and gums with a procedure known as scaling and root planing, a common method of treatment. The medication does not require mixing or refrigeration and is easy to administer. The manufacturer reports that minocycline is painless and easy to apply. The antibiotic does not require dressing or removal because it is completely resorbed and patients can resume routine oral hygiene 12 hours after treatment.
In clinical trials, treatment with minocycline following scaling and root planing significantly reduced pocket depth compared with scaling and root planing alone. This was true even in the sites that were hardest to treat and with the most challenging patients, such as smokers, those with cardiovascular disease and those older than 50 years.
COPYRIGHT 2001 American Academy of Family Physicians
COPYRIGHT 2001 Gale Group