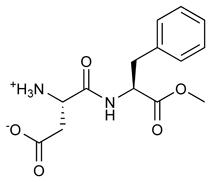
Aspartame
Aspartame is the name for an artificial, non-carbohydrate sweetener, aspartyl-phenylalanine-1-methyl ester; i.e., the methyl ester of the dipeptide of the amino acids aspartic acid and phenylalanine. It is marketed under a number of trademark names, such as NutraSweet, Equal, and Canderel, and is an ingredient of approximately 5,000 consumer foods and beverages sold worldwide. It is commonly used in diet soft drinks, and is often provided as a table condiment. It is also used in some brands of chewable vitamin supplements. more...
However, aspartame is not always suitable for baking, because it often breaks down when heated and loses much of its sweetness. In the European Union, it is also known under the E number (additive code) E951. Aspartame is also one of the sugar substitutes used by diabetics.
Aspartame has been the subject of a vigorous public controversy regarding its safety and the circumstances around its approval. It is well-known that aspartame contains the naturally-occurring amino acid phenylalanine, which is a health hazard to the few people born with phenylketonuria, a genetic inability to process phenylalanine. A few studies have also recommended further investigation into possible connections between aspartame and diseases such as brain tumors, brain lesions, and lymphoma, but no large-scale studies have been conducted. These possibilities, combined with notable conflicts of interest in the approval process, have engendered vocal activism regarding the legitimate risks of aspartame, as well as some less credible theories.
Chemistry
Aspartame is the methyl ester of the dipeptide of the natural amino acids L-aspartic acid and L-phenylalanine. Under strongly-acidic or -alkaline conditions, aspartame first generates methanol by hydrolysis. Under more severe conditions, the peptide bonds are also hydrolyzed, resulting in the free amino acids.
Properties and use
Aspartame's attractiveness as a sweetener comes from the fact that it is approximately 180 times sweeter than sugar in typical concentrations without the high energy value of sugar. While aspartame, like other peptides, has a caloric value of 4 kilocalories (17 kilojoules) per gram, the quantity of aspartame needed to produce a sweet taste is so small that its caloric contribution is negligible, which makes it a popular sweetener for those trying to avoid calories from sugar. The taste of aspartame is not identical to that of sugar: aspartame's sweetness has a slower onset and longer duration than sugar's, and some consumers find it unappealing. Blends of aspartame with acesulfame potassium are purported to have a more sugar-like taste, and to be more potent than either sweetener used alone.
Like many other peptides, aspartame may hydrolyze (break down) into its constituent amino acids under conditions of elevated temperature (in the case of aspartame, 86 °C) or high pH. This makes aspartame undesirable as a baking sweetener, and prone to degradation in high-pH products requiring a long shelf life. Aspartame's stability under heating can be improved to some extent by encasing it in fats or in maltodextrin. Aspartame's stability when dissolved in water depends markedly on pH. At room temperature, it is most stable at pH 4.3, where its half-life is nearly 300 days. At pH 7, however, its half-life is only a few days. Most soft-drinks have a pH between 3 and 5, where aspartame is reasonably stable. In products that may require a longer shelf life, such as syrups for fountain beverages, aspartame is sometimes blended with a more stable sweetener, such as saccharin.
Read more at Wikipedia.org