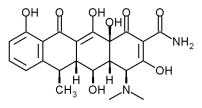
NEWTOWN, Pa. -- New Combination Approach Resulted in up to a 48% Increase in Attachment Level and a 44% Reduction in Periodontal Pocket Depth Compared to SRP Control
CollaGenex Pharmaceuticals, Inc. (Nasdaq:CGPI) today announced the clinically and statistically significant results of a six-month, 180-patient clinical trial designed to evaluate the safety and efficacy of Periostat(R) when combined with Atridox and scaling and root planning (SRP), versus SRP alone. All patients entering the trial had eight diseased tooth sites with periodontal pocket depths of at least 5 mm. At the end of the six month study, patients in the Periostat + Atridox + SRP group showed a 48% mean improvement in clinical attachment level and a 44% mean reduction in periodontal pocket depths for pockets of 7 mm or greater at baseline, compared to comparable pockets in the patients who had SRP alone. Those deep pockets with baseline depths of 7 mm or greater and treated with combination therapy experienced an average reduction in pocket depth of more than 2 mm.
The results, showing that the patients receiving the combination of treatments experienced more than a 2 mm improvement in mean attachment gain and pocket depth reduction, were highly statistically significant when compared to SRP alone (p = less than 0.0001 for both parameters).
"These data clearly show the clinical benefit of combination host modulation and topical anti-microbial therapy when administered along with traditional scaling and root planing," said Dr. John Novak, Director of Clinical Research at the University of Kentucky School of Dental Medicine and lead investigator. "An average reduction of 2 mm or more in deep periodontal pockets can mean the difference between being able to maintain the teeth with conservative, non-surgical therapy versus the use of more aggressive, surgical techniques. After six months of this type of comprehensive, non-surgical approach, more than three times as many patients had no further need for corrective periodontal surgery compared to those patients receiving scaling and root planing alone."
"Periostat has demonstrated an outstanding record of safety and efficacy, with over 3 million prescriptions filled to date," said Colin Stewart, president and chief executive officer of CollaGenex. "The results from this combination trial add to the growing body of evidence supporting the use of Periostat and Atridox to treat this common and potentially debilitating disorder."
CollaGenex Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on providing innovative medical therapies to the dental and dermatology markets. Currently, the Company's professional dental pharmaceutical sales force markets Periostat, which is indicated as an adjunct to scaling and root planing for the treatment of adult periodontitis. Periostat is the first and only pharmaceutical to treat periodontal disease by inhibiting the enzymes that destroy periodontal support tissues, and by enhancing bone protein synthesis. The dental sales force also promotes Atridox(R), Atrisorb FreeFlow(R) and Atrisorb-D FreeFlow(R), Atrix Laboratories, Inc.'s products for the treatment of adult periodontitis, to the dental market. The Company's professional dermatology sales force markets Pandel(R), a prescription topical corticosteriod licensed from Altana, Inc.
Research has shown that certain unique properties of the tetracyclines discovered during the development of Periostat(R) may be applicable to other diseases involving inflammation and/or destruction of the body's connective tissues, including acne, rosacea, meibomianitis and cancer metastases, among others. CollaGenex is further evaluating Periostat(R), as well as the new IMPACS(R) compounds, to assess whether they are safe and effective in these applications. In addition, CollaGenex has licensed the Restoraderm(R) technology, a unique, proprietary dermal drug delivery system, in order to develop a range of topical dermatological products with enhanced pharmacologic and cosmetic properties.
To receive additional information on the Company, please visit our Web site at www.collagenex.com, which does not form part of this press release.
This press release contains forward-looking statements that involve risks and uncertainties. Our business of selling, marketing and developing pharmaceutical products is subject to a number of significant risks, including risks relating to the implementation of CollaGenex's sales and marketing plans for Periostat and other products that we market, risks associated with our arrangement with Mutual, risks inherent in research and development activities, risks associated with enforcement of our intellectual property rights, risks that the FDA will approve products that will compete with and limit the market for Periostat, risks relating to our litigation with the FDA, risks associated with conducting business in a highly regulated environment and uncertainty relating to clinical trials of products under development. CollaGenex's success depends to a large degree upon the market acceptance of Periostat by periodontists, dental practitioners, other health care providers, patients and insurance companies and the success of our dermatology product candidates. There can be no assurance that CollaGenex's product candidates (other than the FDA's approval of Periostat for marketing in the United States, the United Kingdom Medicines and Healthcare products Regulatory Agency's approval of Periostat for marketing in the United Kingdom and Periostat's marketing approval in Austria, Finland, Switzerland, Ireland, Israel, Italy, Luxembourg, the Netherlands, Portugal and Canada) will be approved by any regulatory authority for marketing in any jurisdiction or, if approved, that any such products will be successfully commercialized by CollaGenex. In addition, there can be no assurance that CollaGenex will successfully promote Pandel, Atridox, Atrisorb-FreeFlow or Atrisorb-D. As a result of such risks and those risks set forth in CollaGenex's filings with the Securities and Exchange Commission, CollaGenex's actual results may differ materially from the results discussed in or implied by the forward-looking statements contained herein. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Periostat(R), IMPACS(R), Metastat(R) and Restoraderm(R) are registered trademarks of CollaGenex Pharmaceuticals, Inc.
All other trade names, trademarks or service marks are the property of their respective owners and are not the property of CollaGenex Pharmaceuticals, Inc. or any of our subsidiaries.
Pandel(R) is a trademark of Taisho Pharmaceuticals.
Atridox(R), Atrisorb(R) and Atrisorb-D(R) are registered trademarks of Atrix Laboratories, Inc.
COPYRIGHT 2004 Business Wire
COPYRIGHT 2004 Gale Group