Gingival diseases are the most widely dispersed diseases in the United States. In some patients, periodontal disease appears in a generalized form, but more often it appears in localized areas. Furthermore, after treatment with scaling and root planing in generalized cases, the disease is often reduced to a few local areas in the patient's mouth. Because periodontitis is a bacterial infection with known pathogenic microorganisms, the local delivery of antimicrobial agents has been considered to be a possible solution for treating and controlling localized forms of periodontal disease. Three local chemotherapeutic agents are reviewed in this paper: tetracycline fiber, doxycycline gel, and chlorhexidine chip. With the advancement of local drug delivery systems, restorative dentists, periodontists, and their patients have new alternatives for the treatment of periodontal disease. Local chemotherapeutic agents offer an additional mode of therapy and should be used on a case-by-case basis, not necessarily as an initial treatment.
Introduction
Approximately 80 to 90% of all adults in the United States have experienced some degree of periodontal disease.1 Twenty-six percent of adults have completely lost all of their teeth by 65 years, and 44% have lost them by 75 years.2 Gingival diseases are the most widely dispersed diseases in the United States. Gingivitis has been defined as an inflammation of the gingiva with no loss of clinical attachment.3 Although gingivitis affects millions of Americans, it is a reversible disease. With meticulous oral hygiene, the patient can be restored to optimum gingival health.
Periodontal disease is much more severe. Genco et al.3 define periodontitis as apical migration of the functional epithelium onto the root surface. Hence, there is loss of the periodontal ligament attachment and the alveolar bony support. There are several types of periodontitis classified by the American Academy of Periodontology, although they all have the same characteristic features. The clinical findings include increased probing depth (PD), bleeding on gentle probing (BOP), loss of clinical attachment level (CAL) and alteration in the physiologic contour of the gingiva. Unfortunately, periodontitis cannot be cured, but it can be arrested.
In some patients, periodontal disease appears in a generalized form, but more often it appears in localized areas. Furthermore, after treatment with scaling and root planing (SRP) in generalized cases, the disease is often reduced to a few local areas of the patient's dentition. Because periodontitis is a bacterial infection with known pathogenic microorganisms, the local delivery of antimicrobial agents has been considered as a possible option for treating and controlling localized forms of periodontal disease.
Controlled Local Delivery
Ongoing research in periodontics has led to the advent of controlled local delivery of medication into the periodontal pocket. Local delivery is defined as delivering an agent to the site of required action.4 Controlled delivery provides an agent on a consistent basis, with a standardized dose, for a sufficient duration of time. Two examples of a currently marketed controlled delivery system are the nicotine skin patch to aid in smoking cessation and the scopolamine skin patch for the prevention of motion sickness. Therefore, if a medicament can be applied to the site of required action, on a consistent basis, at a standardized dose, for a sufficient period of time, then controlled local delivery is achieved. When dealing with periodontal disease, controlled local delivery of an antimicrobial agent must also be effective against a target organism at that site of action. Finally, it must cause no harm to the patient. The goal is that pathogens in the periodontal pocket will be suppressed or eradicated by controlled local delivery of the antimicrobial agent.
History
As early as 1979, Goodson and colleaguess used cellulose acetate hollow tubing to deliver tetracycline into periodontal pockets. They discovered that this technique could dramatically reduce periodontal microflora and the gingival index (a measurement of inflammation) as defined by Loe and Silness.6 Unfortunately, the delivery of tetracycline was brief and did not last long enough to be effective. Later that year, Lindhe et al. reported using local delivery of tetracycline via the same hollow fiber.7 With this fiber, it was possible to change the composition of the subgingival flora and reduce the clinical symptoms of periodontal disease.
Introduction of local delivery of tetracycline via a fiber overcame some of the shortfalls of systemic administration, such as systemic side effects, drug interactions, and low sulcular concentration. However, several problems still remained. The fibers were time consuming to place, were often dislodged from the pocket, and were not readily resorbed by the body. Therefore, the patient had to return for fiber removal.
These problems led to the advent of other local chemotherapeutic agents that would be equally as effective and readily resorbed by the body. In 1998, the U.S. Food and Drug Administration approved two local chemotherapeutic agents, Atridox gel and PerioChip (Table I). Both agents are biodegradable and self-retentive, ensuring patient compliance.
Gingival Crevicular Concentration
Gingival crevicular fluid (GCF) is an altered serum transudate released into the gingival sulcus. The flow of this fluid averages approximately 20 (mu)L/h and increases dramatically with gingival inflammation.8 Goodson estimated that the fluid present in a 5-mm periodontal pocket is replaced about 40 times an hour, or once every 1.5 minutes.9 Mean GCF concentration has been measured for each chemotherapeutic agent for up to 1 week (Table II). With the rapid rate of clearance in the periodontal pocket, it becomes important to carry a bacteriocidal or bacteriostatic concentration for at least 7 days.
Local Chemotherapeutic Agents
Currently, there are five local chemotherapeutic agents on the market available for the treatment of periodontal disease (Table 1). Because of the minimal clinical use and unavailability of metronidazole gel and minocycline ointment in the United States, they are excluded from this discussion.
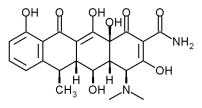
The tetracyclines, tetracycline hydrochloride and doxycycline, are broad-spectrum antibiotics that are effective against anaerobes and facultative organisms. They are bacteriostatic against both Gram-positive and Gram-negative bacteria. Chlorhexidine is an antiseptic known to reduce gingivitis when used as a mouth rinse and also as a subgingival irrigant.10,11
Tetracvcline Fibers (Actisite)
The Actisite delivery system (t-fiber) is made of a monolithic ethylene/vinyl acetate nonresorbable polymer, which contains a 25% saturation of tetracycline for a total of 12.7 mg of evenly dispersed tetracycline hydrochloride. The t-fiber is 230 mm in length and 0.5 mm in diameter, making it small enough to be placed into any periodontal pocket (Figs. 1 and 2). It is flexible and can be folded upon itself to fill the pocket and sealed with a cyanoacrylate adhesive for 7 to 10 days. The tetracycline is released at a constant rate of 2 (mu)g/cm/h for up to 14 days.12 Mean gingival tissue fluid concentration is 1,590(mu)g/mL 13 vs. 4 to 8 (mu)g/mL 14 for systemic administration. The maximum serum concentration is
The disadvantages of using this system are the time needed for insertion (7-10 minutes per tooth) along with the requirement for fiber removal after 10 days. Another disadvantage is bacterial resistance. Lacroix et al.16 found that 12% of the normal periodontal flora are resistant to tetracycline when given systemically. Further research is needed to determine if resistance develops after use of the t-fiber.
Tetracycline fiber was first introduced in 1979 and has undergone many clinical trials. Rapley et al. determined that local delivery of tetracycline could provide and maintain a drug concentration inhibiting periodontal pathogens. I Additionally, they concluded that tetracycline in the periodontal pocket could be adsorbed by the tooth cementum, which would create a repository to stimulate tetracycline's anticollagenase activity. Tetracycline's reservoir effect decreases collagen breakdown and enhances periodontal ligament regeneration." Tetracycline has also been detected in the epithelial tissue after local delivery to a depth of 20 (mu)m.18
Goodson et al.19 published data from a 12-month study to determine whether t-fiber alone was more beneficial than combination therapy of tetracycline and SRP (Table III). The authors reported that with a combination of t-fiber therapy and SRP, attachment gain appeared delayed in contrast to either scaling alone or t-fiber therapy alone. This delay in healing was attributed to the mechanical interference of the t-fiber in the periodontal pocket. Clinical attachment gain after 12 months was slightly better in the combination therapy group than in all other treatment groups. However, this was not statistically significant.
Newman et al.20 conducted a multicenter, longitudinal, randomized study on 113 patients with recurrent periodontitis. They found that PD and BOP reduction was significantly greater at 1, 3, and 6 months when combining t-fiber with traditional SRP compared with SRP alone. Furthermore, CAL was significantly superior at 6 months when combining SRP and the t-fiber compared with SRP alone.
Another multicenter, longitudinal, randomized study by Goodson et al. in 1991 evaluated the t-fiber, a placebo fiber, SRP, and no treatment.21 The authors used a four-quadrant design with 107 patients having pockets 6 mm or greater. After 60 days, PD reduction was 1.02 mm for the t-fiber group, 0.57 for the placebo group, 0.67 for the SRP group, and 0.46 for the nontreatment group. This decrease was statistically significant for the t-fiber group compared with all other groups. Also, for both CAL gain and BOP, the t-fiber group proved statistically superior to the other groups.
Doxycycline Polymer (Atridox)
Atridox is a biodegradable gel containing 10% (w/w) doxycycline, 33% (w/w) poly-DL-lactide , and 57% (w/w) N-methyl-2-- pyrrolidone.22 The medicament is supplied in two syringes that must be mixed together chairside for 25 repetitions (approximately 30 seconds) (Fig. 3). The mixed solution is placed into one syringe to which a 23-gauge cannula is attached and is placed to the depth of the pocket. The solution is expressed until it overfills the pocket and begins to set (Fig. 4). Upon contact with the moist environment, the N-methyl-2-pyrrolidone solvent diffuses out as the liquid poly-DL-lactide rapidly solidifies. The residual polymer can then be packed into the pocket using the underside of a curette. Treatment areas should not be brushed or flossed for 1 week, and the patient is prescribed chlorhexidine mouth rinse twice a day.22
Resistance with doxycycline is lower than with tetracycline. In patients with adult periodontitis, natural resistance is 4.2% of the anaerobic subgingival bacteria.23 Development of bacterial resistance to doxycycline was studied using the Atridox system. The authors validated the development of short-term resistance (7-21 days); however, no long-term change (90-180 days) in the proportion of doxycycline-resistant bacteria in the subgingival flora was noted.24
Several studies have assessed the efficacy of Atridox used as a local delivery antimicrobial agent (Table IV). The first study, published by Polson et al. in 1997, suggests that for PD reduction and gaining CAL, doxycycline hyclate (DH) applied in a vehicle carrier was superior to sanguinarine and the vehicle alone.25 Another study, by Garrett et al. in 1997, showed no statistical significance between DH alone and SRP when evaluating periodontal PD and CAL.26
Recently, Garrett et al. published a study that evaluated the effectiveness of DH in 822 moderate to severe periodontitis patients.27 They compared doxycycline polymer (8.5%) to placebo control, oral hygiene, and SRP in two multicenter sites. After 9 months, the authors concluded that DH alone produced the largest decrease in probing depth at 1.2 mm, compared with the oral hygiene group (0.6 mm), the placebo group (0.8 mm), and the SRP group (1.1 mm). Mean increase in CAL for the DH group was 0.8 mm, superior to the oral hygiene group (0.4 mm), the placebo group (0.4 mm), and the SRP group (0.7 mm).
Chlorhexidine Gluconate (PerioChip)
Chlorhexidine (CHI was introduced in the 1970s as a topical antimicrobial agent.28 Since then, it has developed into a powerful antiseptic capable of reducing plaque by 25 to 40% when used as a rinse or irrigation, respectively.29 CHX has a specific mechanism of action against bacteria.28 The positively charged, long-chain molecule attaches to the negatively charged cell wall of the bacteria, disrupting the cell wall membrane. The cell wall ruptures with loss of the cytoplasm, resulting in cell death.29
The CHX chip is a 4-mm x 5-mm biodegradable film of hydrolyzed gelatin containing 2.5 mg of CHX gluconate (Fig. 5).30 The chip is easily placed into periodontal pockets greater than 5 mm and requires no retentive system (Fig. 6). The body resorbs the chip in 8 to 10 days.
The main adverse effects of CHX rinse are staining of the teeth, calculus formation, and altered taste sensation. However, few anaphylactic and allergic reactions have occurred, mainly in patients of Japanese descent.31 When CHX is used in a chip, minimal side effects are induced. Most notable is a tendency for patients to complain of toothache or tooth sensitivity.28 The CHX chip does not visibly stain the teeth.28
CHX is delivered from the chip into the gingival sulcus at a concentration greater than 125 (mu)g/mL for at least 7 days.32 Several studies have measured CHX concentrations in the GCF. Soskolne et al. reported a mean CHX concentration in the GCF at 4 hours of 1,444 (mu)g/mL; at 72 hours the concentration was 1,900 (mu)g/mL, and at day 7 the concentration was still greater than 125 (mu)g/mL.32 This concentration is high enough to kill pathogenic bacteria that are known to cause periodontal disease. Specifically, at this concentration, the mean percentage of subgingival bacteria inhibited in vitro was 99%.33
Clinical data on the use of the CHX chip are remarkable (Table V). Studies have shown that the CHX chip can significantly improve gingival health when used as an adjunct to SRP.30 The first such study was a European study by Soskolne and colleagues in 1997.34 This 6-month, multicenter, randomized study involved 118 patients with moderate periodontitis. A splitmouth design on the maxillary arch was used to compare the performance of SRP alone vs. SRP with CHX in pockets ranging from 5 to 8 mm. CHX chips were placed at baseline and at 3 months in all pockets greater than 5 mm. Examiners measured PD, CAL, and BOP. The sites treated with SRP and CHX had a significantly greater reduction in average PD at 6 months than the SRP sites alone (1.16 vs. 0.7 mm). Also at 6 months, the CHX group had a significantly greater increase in CAL in pockets >= 7 mm than the SRP alone group (0.98 vs. 0.33 mm). The results of this study demonstrate that treatment of periodontal pockets with CHX and SRP provides a significantly greater improvement in PD and CAL than SRP alone.
Another clinical, randomized, double blind, multicenter study conducted in the United States evaluated the effects of CHX in conjunction with SRP. Jeffcoat et al.35 reported on 447 patients with adult periodontitis divided into two groups: a treatment group (225 patients) and a placebo group (222 patients). After 1 hour of SRP, the CHX chip was placed in target sites with probing depths from 5 to 8 mm. The control group received SRP and a placebo chip. If PD remained >= 5 mm, new CHX or placebo chips were placed at 3 and/or 6 months. Approximately 60% of the patients had a second or third chip placed.
At 9 months, the CHX chip treatment group had significant reductions in PD with respect to the two control groups (CHX + SRP, 0.95 mm; SRP, 0.65 mm; placebo chip + SRP, 0.69 mm). The CHX chip treatment group also showed significant reductions in CAL with respect to the two control groups (CHX + SRP, 0.75 mm; SRP, 0.58 mm; placebo chip + SRP, 0.55 mm). Furthermore, 19% of patients in the CHX chip group experienced a significant PD reduction from baseline of 2 mm or more at 9 months compared with the SRP group (8%).
In a recent study on the effect of alveolar bone height after 9 months of treatment with the CHX chip, Jeffcoat et al.36 evaluated 45 patients with at least four 5- to 8-mm sites. Patients received SRP alone, SRP and placebo chip, or SRP and CHX. Radiographs were taken via quantitative digital subtraction radiography. Interestingly, 25% of sites treated with SRP and the CHX chip experienced bone gain. Conversely, 15% of subjects treated with SRP alone continued to lose bone in one or more sites during the period of the study.35
The trial studies on the CHX chip demonstrate that it is a safe and effective adjunctive chemotherapy for the treatment of periodontal disease. Adverse effects of CHX chip placement have been minimal.
Discussion
Currently, local delivery of antimicrobial agents is relatively new in the field of dentistry. Although the t-fiber has been available since the late 1970s, it has been used infrequently because of the time needed for placement and the additional appointment required for removal. With the advantages of Atridox and PerioChip, periodontal therapy is rapidly becoming more conservative. These systems are easy to place, requiring only a few minutes per tooth, self-retentive, have large a concentration of antimicrobial agent, and are bioabsorbable. Additionally, doxycycline has shown to cause significantly less bacterial resistance than tetracycline.24 Furthermore, CHX is extremely safe, with its only contraindication to use is patients with a known allergy.
Conclusion
With the advancement of local drug delivery systems, restorative dentists, periodontists, and their patients have new alternatives for the treatment of periodontal disease. Local chemotherapeutic agents offer an additional mode of therapy and should be used on a case-by-case basis, not necessarily as an initial treatment. No other treatment has proven as beneficial as oral hygiene instructions and conventional scaling and root planing. For the majority of patients, periodontal sites will respond adequately to scaling and root planing and require no additional therapy. The use of local drug delivery systems in those situations would be considerable overtreatment. Therefore, after thorough scaling and root planing, local antimicrobial therapy should be used after a thorough reevaluation, and only if a possibly exists to reduce the need for periodontal surgery. Moreover, sites that are nonresponsive should be treated with bacterial and antibiotic sensitivity testing to determine which pathogenic microorganisms are present and their susceptibility to antimicrobial agents.
One question that still remains is whether placement of one local delivery agent on one surface of the tooth provides optimal antibacterial concentration circumferentially around the tooth. Further research is needed in this area.
References
1. Brown LJ, Brunelle JA, Kingman A: Periodontal status in the United States, 1988-1991: prevalence, extent, and demographic variation. J Dent Res 1996; 75: 672-83.
2. Marcus SE, Drury TF, Brown IJ, Zion GR: Tooth retention and tooth loss in the permanent dentition of adults: United States, 1988-1991. J Dent Res 1996: 75: 684-95.
3. Genco Rd, Goldman HM, Cohen DW: Contemporary Periodontics, pp 63-72. St. Louis, MO, CV Mosby, 1990.
4. Killoy WJ, Cobb CM: Controlled local delivery of tetracycline in the treatment of periodontitis. Compendium 1992; 12: 1150-8.
5. Goodson JM, Haffajee A, Socransky SS: Periodontal therapy by local delivery of tetracycline. J Clin Periodontol 1979: 6: 83-92.
6. Loe H, Silness J: Periodental disease in pregnancy. I. Prevelance and severity. Acta Odontol Scand 1963; 21: 533-51.
7. Lindhe J, Heijl L, Goodson JM, Socransky SS: Local tetracycline delivery using hollow fiber devices in periodontal therapy. J Clin Periodontol 1979; 6: 141-9.
8. Binder TA, Goodson JM, Socransky SS: Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res 1987; 22: 14-9.
9. Goodson J: Pharmacokinetic principles controlling efficacy of oral therapy. J Dent Res 1989; 68: 1625-32.
10. Aziz-Gandour IA, Newman HN: The effects of a simplified oral hygiene regime plus supragingival irrigation with chlorhexidine or metronidazole on chronic inflammatory periodontal disease. J Clin Periodontol 1986: 13: 228-36.
11. Jolkovsky DL, Waki MY, Newman MG, et al: Clinical and microbiological effects of subgingIval and gingival marginal irrigation with chlorhexidine gluconate. J Periodontol 1990; 61: 663-9.
12. Killoy WJ: Chemical treatment of periodontitis: local delivery of antimicrobials. Int Dent J 1998; 48(suppl 1): 305-15.
13. Tonetti M, Cugini MA, Goodson JM: A zero-order delivery with periodontal placement of tetracycline loaded ethylene vinyl acetate fibres. J Periodontol Res 1990; 25: 243-7.
14. Gordon J, Walker CB, Murphy J, Goodson JM, Socransky SS: Tetracycline levels achievable in gingival crevice fluid and in vitro effect on subgingival organisms. I. Concentrations in crevicular fluid after repeated doses. J Periodontol 1981; 52: 609-12.
15. Rapley JW. Cobb CM, Filloy WJ, Williams DR: Serum levels of tetracycline during treatment with tetracycline containing fibers. J Periodontol 1992; 63: 817-20.
16. Lacroix JM, Walker CB: Detection and incidence of the tetracycline resistant determinant tet (in) in the microflora associated with adult periodontitis. J Periodontol 1995; 66: 102-8.
17. Slots J, Ram T: Antibiotics in periodontal therapy: advantages and disadvantages. J Clin Periodontol 1990: 17: 479-93.
18. Cianco SG, Cobb CM, Leung M: Tissue concentration and localization of tetracycline following site specific tetracycline fiber therapy. J Periodontol 1992; 63: 849-53.
19. Goodson JM, Hogan PE, Dunham SL: Clinical responses following periodontal treatment by local drug delivery. J Periodontol 1985: 56(suppl): 81-7.
20. Newman MG, Kornman KS, Doherty FM: A 6-month multi-center evaluation of adjunctive tetracycline fiber therapy used in conjunction with scaling and root planing in maintenance patients: clinical results. J Periodontol 1994; 65: 68591.
21. Goodson JM, Cugini MA, Dent RL, et al: Multi-center evaluation of tetracycline fiber therapy. II. Clinical response. J Periodontol Res 1991; 26: 371-9.
22. Johnson LR, Stoller NH: Rationale for the use of Atridox therapy for managing periodontal patients. Compendium 1999; 20: 19-25.
23. Borden LC, Walker CB, Godowski KC: Prevalence of doxycycline-resistant bacteria in an adult periodontitis population [abstract 2425(. J Dent Res 1996: 75: 321.
24. Borden LC, Walker CB, Stone C, Manxodi S, Godowski KC, Southard GL: Microbiota effects following sustained release subgingival delivery of doxycycline [abstract 1141. J Dent Res 1997; 76: 153.
25. Poison AM, Garrett S, Stoller NH, et al: Multi-center comparative evaluation of subgingivally delivered sanguinarine and doxycycline in the treatment of periodontitis. II. Clinical results. J Periodontol 1997: 68: 119-26.
26. Garrett S, Adams D, Bandt C, et al: Two multicenter clinical trials of subgingival doxycycline in the treatment of periodontitis [abstract 11131. J Dent Res 1997; 76: 153.
27. Garrett S, Johnson L, Drisko CH, et al: Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol 1999; 70: 490503.
28. Ciancio SG: Local delivery of chlorhexidine. Compendium 1999; 20: 427-33.
29. Fleming TF, Newman MG, Doherty FM, Grossman E, Meckel AH, Bakdash B: Supragingival irrigation with 0.06% chlorhexddine in naturally occurring gingivitis. I. 6 month clinical observations. J Periodontol 1990; 51: 112-7.
30. Killoy WJ: Assessing the effectiveness of locally delivered chlorhexidine in the treatment of periodontitis. J Am Dent Assoc 1999; 130: 567-70.
31. Okano M, Nomuar M, Hata S, et al: Anaphylactic symptoms due to chlorhexidine gluconate. Arch Dermatol 1989; 125: 50-2.
32. Soskolne WA, Chajek T, Flashner M, et al: An in vivo study of the chlorhexidine
release profile of the PerlioChipTM in the gingival crevicular fluid, plasma and urine. J Clin Pertodontol 1998: 25: 1017-21.
33. Stanley A, Wilson M, Newman HN: The in vitro effects of chlorhexidine on subgingival plaque bacteria. J Clin Periodontol 1989; 16: 259-64.
34. Soskolne WA, Heasman PA, Stabholz A, et al: Sustained local delivery of chlorhe)ddine in the treatment of periodontitis: a multi-center study. J Periodontol 1997: 68: 32-8.
35. Jeffcoat MK, Bray KS. Cianco SG, et at: Adjunctive use of a subgingival controlledrelease chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol 1998: 69: 989-97.
36. Jeffcoat MY. Palcanis KG, Weatherford IV, Reese M, Geurs NC, Flashner M: Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: clinical and radiographic findings. J Periodontol 2000; 71: 256-62.
37. Stoller NH, Johnson LR, Trapnell S, Han-old CQ, Garrett S: The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J Periodontol 1998; 69: 1085-91.
Guarantor: CPT Dennis S. Norkiewicz, DC USA
Contributors: CPT Dennis S. Norkiewicz, DC USA; LTC(P) Lawrence G. Breault, DC USA; COL Steven T. Wonderlich, DC USA; LTC(P) Kay H. Malone, DC USA
Schofield Barracks Dental Clinic, Schofield Barracks. HI.
This manuscript was received for review in November 2000. The revised manuscript was accepted for publication in May 2001.
Reprint & Copyright Oc by Association of Military Surgeons of U.S., 2001.
Copyright Association of Military Surgeons of the United States Nov 2001
Provided by ProQuest Information and Learning Company. All rights Reserved