Business Editors/Health & Medical Writers
NEWTOWN, Pa. & FORT COLLINS, Colo.--(BUSINESS WIRE)--Nov. 1, 2001
CollaGenex Pharmaceuticals, Inc. (Nasdaq:CGPI) today announced that it has shipped and recorded its first sales of Atrix Laboratories, Inc. (Nasdaq:ATRX) proprietary dental products, Atridox(R) and Atrisorb(R)-FreeFlow, to the U.S. dental market.
In August 2001, CollaGenex signed an exclusive licensing and marketing agreement with Atrix to market these products to the dental community. CollaGenex is the leading specialty dental pharmaceutical company with a professional sales force of approximately 120 sales representatives and managers.
The CollaGenex sales force has been extensively trained in the technical and sales aspects of the Atrix dental products since early October. The dental products are manufactured by Atrix with the CollaGenex trade dress, and shipments of these products were initiated on November 1.
"We believe our existing customer base will respond very favorably to the introduction of these products by CollaGenex's sales force," said Brian M. Gallagher, PhD, chairman, president and chief executive officer of CollaGenex. "Initial indications suggest a substantial interest in Atridox from a number of our 35,000 dental customers, many of whom had not been targeted by prior sales and marketing efforts. There also appears to be a clear recognition that the Atridox clinical data and label are far superior to other locally-applied antimicrobial agents, which we believe provides an opportunity for CollaGenex to increase sales of Atridox significantly."
"We have received anecdotal reports from our sales force and target dentists that patients have obtained excellent clinical results from the two-pronged approach of using Periostat(R), our proprietary enzyme suppressor, and Atridox, the leading locally applied antimicrobial agent, along with traditional approaches to therapy in the treatment of their periodontitis," Dr. Gallagher continued. "We have also seen significant interest in Atrisorb-D, and we remain enthusiastic about launching this novel product early in 2002."
"We are pleased with the enthusiasm of the CollaGenex sales force for our dental products and the professionalism that they bring to the sales of prescription dental products," said Mr. David Bethune, Atrix's chairman and chief executive officer. "We believe CollaGenex has the drive and the energy to make this collaboration a total success."
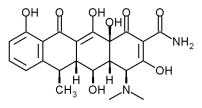
Atridox uses Atrix's patented drug delivery technology, Atrigel(R), for targeted delivery of the antibiotic doxycycline, which has been shown to reduce the levels of bacteria in the periodontal pocket. Atrisorb-D, the first guided tissue regeneration (GTR) barrier product to incorporate an antibiotic, also uses doxycycline to reduce the incidence of undesirable infections during GTR procedures.
Periostat is a pharmaceutical tablet that is administered systemically at a sub-antimicrobial dose to reduce elevated enzyme levels in the gum tissues that cause the tissue destruction characteristic of adult periodontitis. Therefore, dentists will be equipped with both a local anti-microbial and a systemic enzyme-lowering therapy to use along with traditional approaches to periodontal disease management, thereby optimizing patient outcomes.
Atrix Laboratories, Inc. is an emerging specialty pharmaceutical company focused on advanced drug delivery. With five unique patented technologies, Atrix is currently developing a diverse portfolio of proprietary products, including oncology, pain management, and dermatology products. The company also partners with large pharmaceutical and biotechnology companies to apply its proprietary technologies to new chemical entities or to extend the patent life of existing products.
Atrix has strategic alliances with several pharmaceutical companies including collaborations with Pfizer, Elan Corporation plc, Sanofi-Synthelabo, Fujisawa Healthcare Inc., CollaGenex Pharmaceuticals, Inc., MediGene AG, and the Novartis company - Geneva Pharmaceuticals, to use its drug delivery technologies and expertise in the development of new products. Additional information is available on the Atrix Laboratories, Inc. Web site at http://www.atrixlabs.com.
CollaGenex Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on providing innovative medical therapies to the dental market. The Company's lead product, Periostat, was approved by the FDA in September 1998 and is the first and only pharmaceutical to treat periodontal disease by inhibiting the enzymes that destroy periodontal support tissues. In February 2001, the FDA granted marketing approval for a new tablet formulation for Periostat.
Periostat is marketed to the dental community by CollaGenex through a professional pharmaceutical sales force. Currently, the Company's dental sales force is also marketing Vioxx(R), a Merck & Co. drug that CollaGenex co-promotes for the treatment of acute dental pain, and Dentaplex(TM), a unique formulation of vitamins and mineral supplements developed by CollaGenex and MediNiche, Inc. to promote oral health.
Research has shown that the enzyme suppression technology underlying Periostat may also be applicable to other diseases involving destruction of the body's connective tissues, including cancer metastases (Metastat) and a broad range of inflammatory diseases.
CollaGenex is developing a series of novel, proprietary compounds known as IMPACS (Inhibitors of Multiple Proteases and Cytokines) to address these applications. The Company intends to pursue further research and development of these technologies primarily through partnerships with third parties.
This news release contains forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended. Investors are cautioned that forward-looking statements involve risks and uncertainties, which may affect the Company's business and prospects.
The Company's business of selling, marketing and developing pharmaceutical products is subject to a number of significant risks, including risks relating to the implementation of the Company's sales and marketing plans for Periostat; risks inherent in research and development activities; risks associated with conducting business in a highly regulated environment and uncertainty relating to clinical trials of products under development, all as discussed in the Company's periodic filings with the US Securities and Exchange Commission.
To receive additional information on the Company, please visit our Web site at www.collagenex.com, which is not a part of this press release.
Periostat(R), Metastat(R), IMPACS(R) and Dentaplex(TM) are trademarks of CollaGenex Pharmaceuticals, Inc.
CollaGenex(R) and Periostat(R) are trademarks of CollaGenex International Limited.
VIOXX(R)is a trademark of Merck & Co., Inc.
Atridox(R), Atrisorb(R)-FreeFlow and Atrisorb(R)-D are trademarks of Atrix Laboratories, Inc.
COPYRIGHT 2001 Business Wire
COPYRIGHT 2001 Gale Group