Business Editors
NEWTOWN, Pa.--(BUSINESS WIRE)--Aug. 27, 2001
Products Expected to be Accretive to Earnings in 2002
CollaGenex Pharmaceuticals, Inc. (Nasdaq:CGPI) today announced that it has signed an exclusive licensing and marketing agreement with Atrix Laboratories, Inc. (Nasdaq:ATRX) to market Atrix's proprietary dental products, Atridox(R), Atrisorb(R)-FreeFlow and Atrisorb(R)-D, to the U.S. dental market.
Under the terms of the agreement, Atrix will manufacture the dental products for CollaGenex at an agreed transfer price and will receive royalties on future net sales. CollaGenex paid a $1 million upfront licensing fee for the rights to market the products. In addition, Atrix purchased 330,556 newly issued shares of CollaGenex common stock at a premium to the market price. The proceeds from the share purchase will be used primarily to fund a strengthened marketing campaign for Atridox and to launch the Atrisorb-D product.
"Atridox is the market leader for locally-applied anti-microbial therapies to treat adult periodontitis. Importantly, Atridox is a complementary therapy to our lead product, Periostat(R), and represents an important addition to our expanding portfolio of innovative therapeutic products for the dental market," said Brian M. Gallagher, PhD, chairman, president and chief executive officer of CollaGenex.
"For the trailing twelve months, sales of the Atrix dental products were approximately $7 million. As a result of our growing Periostat franchise and 120-person specialized dental sales force, we are uniquely positioned to apply additional resources that will leverage the Atrix family of therapeutic products and broaden their marketability. We expect to begin actively marketing these products in October. Sales of the Atrix dental products should contribute to our growing revenue base, starting in the fourth quarter of this year. With a solid infrastructure to market these products already in place, we expect these products to make a significant contribution to earnings in 2002 and beyond."
"CollaGenex has a proven track record of success in the dental pharmaceutical arena, and we are excited that the premier U.S. dental sales and marketing force will be responsible for the continued growth of our dental products," said David R. Bethune, chairman and chief executive officer of Atrix Laboratories. "We believe Atridox is capable of achieving higher utilization and that CollaGenex, with its established dental sales force, is uniquely qualified to achieve this objective. Furthermore, this provides an opportunity for the therapeutic synergies that we believe exist between Atridox and Periostat to be fully explored for the first time."
Atridox uses Atrix's patented drug delivery technology, Atrigel(R), for targeted delivery of the antibiotic doxycycline, which has been shown to reduce the levels of bacteria in the periodontal pocket. Atrisorb-D, the first guided tissue regeneration (GTR) barrier product to incorporate an antibiotic, also uses doxycycline to reduce the incidence of undesirable infections during GTR procedures.
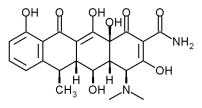
Periostat is a pharmaceutical tablet that is administered systemically at a sub-antimicrobial dose to reduce elevated enzyme levels in the gum tissues that cause the tissue destruction characteristic of adult periodontitis. Therefore, dentists will be equipped with both a local anti-microbial and a systemic enzyme-lowering therapy to use along with traditional approaches to periodontal disease management, thereby optimizing patient outcomes.
CollaGenex Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on providing innovative medical therapies to the dental market. The Company's lead product, Periostat, was approved by the FDA in September 1998 and is the first and only pharmaceutical to treat periodontal disease by inhibiting the enzymes that destroy periodontal support tissues. In February 2001, the FDA granted marketing approval for a new tablet formulation for Periostat.
Periostat is marketed to the dental community by CollaGenex through a professional pharmaceutical sales force composed of approximately 120 sales representatives and managers. Currently, the Company's dental sales force is also marketing Vioxx(R), a Merck & Co. drug that CollaGenex co-promotes for the treatment of acute dental pain, and Dentaplex(TM), a unique formulation of vitamins and mineral supplements developed by CollaGenex and MediNiche, Inc. to promote oral health.
Research has shown that the enzyme suppression technology underlying Periostat may also be applicable to other diseases involving destruction of the body's connective tissues, including cancer metastases (Metastat) and a broad range of inflammatory diseases. CollaGenex is developing a series of novel, proprietary compounds known as IMPACS (Inhibitors of Multiple Proteases and Cytokines) to address these applications. The Company intends to pursue further research and development of these technologies primarily through partnerships with third parties.
CollaGenex will host a conference call at 11:00 a.m. today, Monday, August 27th, to discuss this transaction. The dial-in numbers are 800/553-0349 in the U.S. or 612/332-0820 internationally. The call may be accessed via the Internet through www.collagenex.com or www.streetevents.com.
A replay of the call will be available beginning at 2:00 p.m. on August 27, 2001 through September 17, 2001 at 11:59 p.m. by dialing 800/475-6701 in the U.S. or 320/365-3844 Internationally and entering Access Code: 601125. The conference call will also be available for replay on both websites for a period of 90 days.
This news release contains forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended. Investors are cautioned that forward-looking statements involve risks and uncertainties, which may affect the Company's business and prospects.
The Company's business of selling, marketing and developing pharmaceutical products is subject to a number of significant risks, including risks relating to the implementation of the Company's sales and marketing plans for Periostat; risks inherent in research and development activities; risks associated with conducting business in a highly regulated environment and uncertainty relating to clinical trials of products under development, all as discussed in the Company's periodic filings with the US Securities and Exchange Commission.
To receive additional information on the Company, please visit our Web site at www.collagenex.com, which is not a part of this press release.
Periostat(R), Metastat(R), IMPACS(R) and Dentaplex(TM) are trademarks of CollaGenex Pharmaceuticals, Inc.
CollaGenex(R) and Periostat(R) are trademarks of CollaGenex International Limited.
VIOXX(R)is a trademark of Merck & Co., Inc.
Atridox(R), Atrisorb(R)-FreeFlow and Atrisorb(R)-D are trademarks of Atrix Laboratories, Inc.
COPYRIGHT 2001 Business Wire
COPYRIGHT 2001 Gale Group