Purpose: To determine the effect ipratropium bromide nasal spray has on methacholine challenge testing for airway hyperresponsiveness.
Materials and methods: Ten subjects with known airway hyperresponsiveness to methacholine who had been clinically stable in the preceding 2 months participated in a randomized, double-blind, placebo-controlled, crossover study. Methacholine challenge testing was conducted on successive days: day 1 after pretreatment with aqueous 0.03% nasal ipratropium, and day 2 with normal saline solution placebo.
Results: The provocative concentration of methacholine causing a 20% fall in FE[V.sub.1] (P[C.sub.20]) was higher after nasal ipratropium than after saline solution placebo (2.1 mg/mL vs 1.6 mg/mL, p = 0.02). This difference is equal to approximately one-half concentration difference, probably within the limits of reproducibility of the test.
Conclusions: Pretreatment with nasal ipratropium results in a small increase in P[C.sub.20]. Although this difference achieves statistical significance, it is probably not clinically significant.
Key words: airway hyperresponsiveness; ipratropium bromide nasal spray; methacholine challenge test
Abbreviations: MCCT = methacholine challenge test; MDI = metered-dose inhaler; P[C.sub.20] = provocative concentration of methacholine causing a 20% fall in FE[V.sub.1]
**********
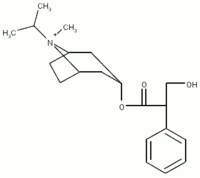
Ipratropium bromide is a quaternary anticholinergic medication that is mostly useful in COPD. Although ipratropium has a limited role in the treatment of asthma, it is a potent antagonist of methacholine, (1-5) the most commonly used agent in bronchoprovocation testing for evaluation of possible asthma. As such, pretreatment with inhaled ipratropium can significantly alter the results of a methacholine challenge test (MCCT) and decrease the sensitivity of the test. Ipratropium is also available as a nasal spray and is very effective at decreasing nasal secretions in patients with allergic or nonallergic rhinitis. (6-10) Nasal ipratropium has been well demonstrated to reduce the effect of methacholine on nasal mucosal secretions. (11-17) Rhinitis and asthma are conditions that commonly coexist. Therefore, patients undergoing an MCCT for asthma evaluation may be using ipratropium nasal spray for rhinitis. Testing guidelines usually recommend that ipratropium by metered-dose inhaler (MDI) or nebulizer be withheld 12 to 24 h prior to MCCT (1,2) to minimize the effect of the drug on test results. No guidelines exist for nasal ipratropium, and it is not known if it has any appreciable effect on the provocative concentration of methacholine causing a 20% fall in FE[V.sub.1] (P[C.sub.20]). We therefore evaluated the effect of a single dose of nasal ipratropium on P[C.sub.20] in patients with known airway hyperresponsiveness to methacholine.
MATERIALS AND METHODS
Subjects
Subjects with known airway hyperresponsiveness to methacholine were recruited through local advertising and by word of mouth. All patients had the clinical diagnosis of asthma, and none had COPD. Nine of the subjects used only intermittent salbutamol (albuterol in the United States) via MDI on an as-needed basis. One of the subjects used regular fluticasone via MDI at a dosage of 250 [micro]g/d plus as-needed salbutamol (Table 1). They were required to have been clinically stable and to have had no change in respiratory medications in the preceding 2 months. A certificate of approval was obtained from the University of Saskatchewan Biomedical Research Ethics Board prior to study initiation. Informed consent was signed by each subject prior to enrollment.
Design
Subjects were evaluated in a double-blind, placebo-controlled, cross-over manner. Active drug and placebo were contained in identical bottles marked as either "A" or "B" and without any other identifiable markers on them. Testing was done on two successive days: day 1 after pretreatment with ipratropium, and day 2 after saline solution placebo. The order in which ipratropinm or placebo was administered was randomly determined. The time interval between pretreatment and MCCT was 10 min.
Methacholine Challenge
Subjects were asked to rest quietly in the laboratory for 10 min prior to the MCCT. Ten minutes prior to testing, subjects instilled two squirts of either 0.03% aqueous nasal ipratropium or placebo (sterile saline solution) into each nostril. The MCCT was performed by the tidal breathing method (1,2) utilizing a jet nebulizer (Bennett Twin; Puritan Bennett; Carlsbad, CA). Methacholine was mixed by the hospital pharmacy from commercially available methacholine chloride powder for inhalation and a combination of 0.9% normal saline solution and 1.5% benzyl alcohol. This is in accordance with the usual protocol at our institution. Methacholine chloride powder for inhalation (Provocholine; Methapharm; Brantford, ON, Canada) is approved for human use by Health Canada.
Analysis
The geometric means for P[C.sub.20] with placebo and ipratropium were compared, using a paired t test; analysis was performed using statistical software (STATISTIX for Windows; Analytical Software; Tallahassee, FL). Power analysis revealed that 10 subjects would be sufficient to detect a one-half concentration difference in P[C.sub.20] (p < 0.05).
RESULTS
Subject characteristics are listed in Table 1. The geometric mean P[C.sub.20] after instillation of placebo nasal spray was 1.6 mg/mL. This increased to 2.1 mg/mL after instillation of two squirts of 0.03% aqueous ipratropium into each nostril (Fig 1). The difference equates to one half of a concentration change (p = 0.02).
[FIGURE 1 OMITTED]
DISCUSSION
The MCCT is useful in the evaluation of possible asthma in a patient with normal pulmonary function test results but suggestive symptoms. The value of the MCCT lies in its excellent sensitivity, allowing a normal test under appropriate conditions to effectively exclude current asthma as the diagnosis. (1,2) Because the purpose of the MCCT is mainly to exclude asthma, conditions that decrease the response to methacholine must be avoided. To that end, the American Thoracic Society has published guidelines (1) regarding medication restriction prior to the MCCT. Inhaled ipratropium bromide is a potent inhibitor of methacholine-induced bronchoconstriction and should be withheld for at least 12 and possibly as long as 24 h prior to testing. (1,2)
Ipratropium nasal spray is indicated for the treatment of rhinitis. As rhinitis is common in patients with asthma, it is likely that some patients undergoing an MCCT may also be using ipratropium nasal spray. Rhinitis and postnasal drip are known to potentially exacerbate asthma and can influence the results on bronchoprovocation testing such as the MCCT. In addition to improving rhinitis, it is possible that nasal ipratropium has direct effects on the MCCT, either through airway deposition or systemic absorption of the medication. Although ipratropium nasal spray has been well demonstrated to decrease nasal secretions induced by nasal methacholine challenge, we could find no study demonstrating the effect nasal ipratropium has on bronchoreactivity during an inhaled MCCT. There is no guideline regarding withholding nasal ipratropium prior to MCCT.
While it seems reasonable to ask patients to withhold from nasal ipratropium for 12 to 24 h prior to MCCT, is it necessary to order repeat testing on an individual who has not done so and has a negative result? Our study demonstrated a statistically significant increase in P[C.sub.20] after nasal ipratropium compared to saline solution. However, the reproducibility of the MCCT is limited to within one doubling dose, and therefore a one-half concentration difference in P[C.sub.20] is likely not clinically significant. Historical data in our laboratory regarding "day-to-day" intraindividual variability indicates a mean absolute difference in P[C.sub.20] of slightly less than one-half concentration, which is approximately the difference we detected between ipratropium and placebo. Based on these results, it seems reasonable to order repeat testing only in patients with borderline results on the MCCT. However, for a patient with a clearly negative result (> 32 mg/mL and probably 16 to 32 mg/mL), repeat testing is unlikely to result in any clinically important difference.
REFERENCES
(1) Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med 2000; 161:309-329
(2) Juniper EF, Cockcroft DW, Hargreave FE. Histamine and methacholine inhalation tests: tidal breathing method; laboratory procedure and standardization. 2nd ed. Lund, Sweden: Astra Draco AB, 1994
(3) Cockcroft DW, Killian DN, Mellon JJ, et al. Protective effects of drugs on histamine induced asthma. Thorax 1977; 32:429-437
(4) Crimi N, Palermo F, Oliveri R, et al. Protective effects of inhaled ipratropium bromide on bronchoconstriction induced by adenosine and methacholine in asthma. Eur Respir J 1992; 5:560-566
(5) Greenspon LW, Morrissey WL. Factors that contribute to inhibition of methacholine-induced bronchoconstriction. Am Rev Respir Dis 1986; 133:735-739
(6) Watase T, Okuda M. The effects of autonomotropic drugs on allergic nasal mucosa. Rhinology 1986; 24:181-186
(7) Sjogren I, Juhasz J. Ipratropium in the treatment of patients with perennial rhinitis. Allergy 1984; 39:457-461
(8) Malmberg H, Grahne B, Holopainen E, et al. Ipratropium (Atrovent) in the treatment of vasomotor rhinitis of elderly patients. Clin Otolaryngol 1983; 8:273-276
(9) Kirkegaard J, Mygind N, Molgaard F, et al. Ordinary and high-dose ipratropium in perennial nonallergic rhinitis. J Allergy Clin Immunol 1987; 79:585-590
(10) Meltzer EO. Intranasal anticholinergic therapy of rhinorrhea. J Allergy Clin Immunol 1992; 90:1055-1064
(11) Becker B, Borum S, Nielsen K, et al. A time-dose study of the effect of topical ipratropium bromide on methacholine-induced rhinorrhea in patients with perennial non-allergic rhinitis. Clin Otolaryngol 1997; 22:132-134
(12) Sjogren I, Jonsson L, Koling A, et al. The effect of ipratropium bromide on nasal hypersecretion induced by methacholine in patients with vasomotor rhinitis: a double-blind, cross-over placebo-controlled and randomized dose-response study. Acta Otolaryngol 1988; 106:453-459
(13) Baroody FM, Majchel AM, Roecker MM, et al. Ipratropium bromide (atrovent nasal spray) reduces the nasal response to methacholine. J Allergy Clin Immunol 1992; 89:1065-1075
(14) Borum S, Becker B, Mygind N, et al. Comparison between the effect of ipratropium bromide as a pressurized aerosol and as an aqueous pump spray on methacholine-induced rhinorrhea. Rhinology 1996; 34:198-200
(15) Wagenmann M, Baroody FM, Jankowski R, et al. Onset and duration of inhibition of ipratropium bromide nasal spray on methacholine-induced nasal secretions. Clin Exp Allergy 1994; 24:288-290
(16) Sanwikarja S, Schmitz PI, Dieges PH. The effect of locally applied ipratropium aerosol on the nasal methacholine challenge in patients with allergic and non-allergic rhinitis. Ann Allergy 1986; 56:162-166
(17) Naclerio RM, Baroody FM. Response of nasal mucosa to histamine or methacholine challenge: use of a quantitative method to examine the modulatory effects of atropine and ipratropium bromide. J Allergy Clin Immunol 1992; 90:1051-1054
* From the Division of Respiratory, Critical Care, and Sleep Medicine, University of Saskatchewan, Saskatoon, SK, Canada. Atrovent was provided by Boehringer Ingelheim Canada.
Manuscript received December 17, 2004; revision accepted February 1, 2005.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal. org/misc/reprints.shtml).
Correspondence to: Donald W. Cockcroft, MD, Division of Respiratory, Critical Care, and Sleep Medicine, University of Saskatchewan, 5th Floor, Ellis Hall, 103 Hospital Dr, Saskatoon, SK, Canada S7N 0W8; e-mail: cockcroft@sask.usask.ca
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group