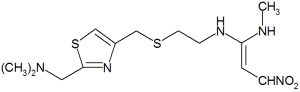
The bioequivalence of nizatidine (Axid) in two extemporaneously and one commercially prepared oral liquid formulations compared with capsule. Abdel-Rahman SM, Johnson FK, Gauthier-Dubois G et al. J Clin Pharmacol 2003;43:148-153.
Nizatidine is an H^sub 2^-receptor antagonist commonly employed in the treatment of acid-related GI disorders when nonpharmacological treatments fail. Oral-liquid dosage formulations provide an alternative for patients who have difficulty swallowing solid oral dosage forms (pediatric and geriatric patients) and subsequently improve patient compliance. An open-label, single dose, four-way crossover trial was used to compare the bioequivalence of two extemporaneous preparations (nizatidine solution in apple juice and nizatidine suspension in infant formula) and one commercially prepared oral-syrup formulation with a commercially prepared oral-solid dosage form (Axid 150-mg capsule). Preparation of the two extemporaneous formulations is described. Venous blood was sampled, and "nizatidine was quantitated from plasma using validated high-performance liquid chromatography assay with mass spectrometric detection." It was found that the commercially prepared oral syrup and the extemporaneously prepared suspension in infantformula were "...bioequivalent to the reference capsule." Nizatidine in apple juice had a "...marked reduction in the extent of bioavailability."
Copyright International Journal of Pharmaceutical Compounding Nov/Dec 2003
Provided by ProQuest Information and Learning Company. All rights Reserved