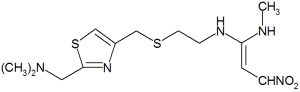
The Food and Drug Administration has given tentative approval to Ivax Corp.'s ANDA for nizatidine capsules USP in strengths of 150 mg and 300 mg. Ivax said its ANDA for nizatidine is the first filed with the agency and, as such, would entitle IVAX to 180 days of market exclusivity. Nizatidine is the generic equivalent of Axid, marketed by Eli Lilly for the relief of heartburn, acid indigestion and sour stomach. Axid had sales of approximately $310 million in 1998. The FDA approval will become final upon the earlier of 300 months from the Nov. 30, 1998, notification to Eli Lilly of the ANDA filing, or the issuance of a decision favorable to IVAX in pending patent litigation. Nizatidine will be marketed by IVAX's wholly owned subsidiary, Zenith Goldilne Pharmaceuticals Inc.
COPYRIGHT 1999 Lebhar-Friedman, Inc.
COPYRIGHT 2000 Gale Group