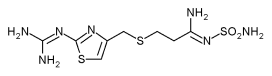
Famotidine
Famotidine is a histamine H2-receptor antagonist that inhibits stomach acid production, and is commonly used in the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD). more...
Clinical use
Certain preparations of famotidine are available over the counter (OTC) in various countries. In the United States, preparations of 10 mg and 20mg tablets, sometimes in combination with a more traditional antacid (brand name Pepcid Complete), are available OTC. Larger doses still require a prescription.
History and development
Famotidine was developed by Merck & Co.. The imidazole-ring of cimetidine was replaced with a 2-guanidinothiazole ring. Famotidine proved to be 30 times more active than cimetidine.
It was first marketed (as Pepcidine® and Pepcid®) in 1985. Pepcid RPD® (orally disintegrating tablets that are not swallowed) was released in 1999. Generic forms (e.g. Fluxid®, Schwarz Pharma) became available in 2001.
In the United States, a product called Pepcid Complete is available that combines famotidine with an antacid in a chewable tablet to ameliorate the relatively slow onset of effects.
Read more at Wikipedia.org