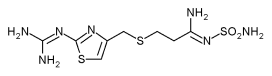
The prescription drug Pepcid (famotidine), used for the short-term treatment of heartburn and peptic ulcers, is now available in generic form. The FDA approved more than two dozen companies' generic versions in April and May, following the end of Pepcid's patent protection, held by Merck Laboratories, on April 15.
The FDA also approved in May one generic version of over-the-counter Pepcid-AC tablets.
Famotidine is available as a tablet and an injection. The drug treats heartburn and non-cancerous ulcers of the digestive system by reducing the amount of stomach acid produced. Felt as a burning sensation in the chest, heartburn is caused by stomach acid eating away at the esophagus.
Heartburn is not life-threatening, but it may be a sign of a more serious problem, such as gastroesophageal reflux disease (GERD). GERD occurs when the muscle valve at the lower end of the esophagus malfunctions, allowing stomach acid to flow up into the esophagus. Left untreated, GERD can cause complications such as Barrett's esophagus (a pre-cancerous condition of the esophagus).
An estimated 15 million Americans experience heartburn every day, according to the American College of Gastroenterology.
We're eager to hear what you like and what you don't like. We also want to know the subjects you'd like to see covered. Letters to the editor can be e-mailed to FDACletters@oc.fda.gov, or mailed to FDA Consumer, Food and Drug Administration (HFI-40),. 5600 Fishers Lane, Rockville, MD 20857. Letters should be 300 words or less, signed, and include an address and telephone number for verification. The editor reserves the right to edit letters for space and appropriateness.
COPYRIGHT 2001 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group