Newer antiepileptic drugs may
control seizures more effectively,
but their significant
potential for serious side
effects requires a thorough
knowledge of the drugs and careful
consideration of the risks and benefits.
Gabapentin
Gabapentin (Neurontin) has been approved
as adjunctive therapy in adults with partial
seizures with or without secondary generalization
(Table 1). A gamma-aminobutyric acid
(GABA) analog, gabapentin does not interact
with GABA receptors. Its mechanism of action is
unknown.
TABLE 1
Comparison of Newer anticonvulsant Drugs
and 400-mg capsules[3] (Table 1). The average
cost of 30 days of treatment at the lowest
recommended dosage is approximately $88.[6]
Gabapentin may be used as adjunctive therapy
in adults with poorly controlled partial seizures.
In the future, gabapentin may become first-line
therapy in patients with newly diagnosed
epilepsy. At present, it has not been approved by
the US. Food and Drug Administration for use
in children. Gabapentin is easy to use and has
relatively mild side effects. Lack of drug-drug
interactions make it an attractive therapy.
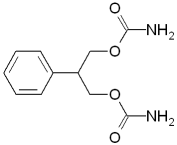
Lamotrigine
Lamotrigine (Lamictal) is included in the
phenyltriazine class. It is used as adjunctive
therapy or monotherapy in adults with partial
seizures with or without secondary generalization.
The mechanism of action is unknown.
Lamotrigine has been shown to act at
voltage-sensitive sodium channels, stabilizing
neural membranes and inhibiting the release
of excitatory neural transmitters.[2]
Lamotrigine is well absorbed orally, with up
to 98 percent bioavailability. Absorption is not
affected by food. Approximately 55 percent of
the drug is protein bound; therefore, clinical
interaction with other protein-bound drugs is
unlikely. Ninety percent of the drug undergoes
glucuronic acid conjugation in the liver,
with the conjugate and the remaining 10 percent
of unmetabolized drug excreted in the
urine.[7] Clearance is markedly increased by the
co-administration of other antiepileptic drugs
that induce hepatic enzymes. These include
carbamazepine (Tegretol), phenobarbital,
phenytoin (Dilantin) and primidone (Mysoline).
The half-life of lamotrigine may be
reduced by about 50 percent with concomitant
use of one or more of these medications
(Tables 2 and 3). However, when combined
with valproic acid, its elimination is decreased,
and its half-life may be more than doubled.
TABLE 3
Effects of Standard Anticonvulsants on Drug Levels of Newer
Anticonvulsants
patients taking felbamate will have aplastic
anemia. The fatality rate of this complication
approaches 30 percent. Aplastic anemia
may not manifest itself until several months
after initiation of treatment, and patients
may remain at risk for an undetermined
amount of time after treatment is discontinued.
The syndrome may begin without
warning and may not be reliably detected by
routine testing. Patients taking felbamate
should remain alert for signs of infection,
bleeding and easy bruising, or symptoms of
anemia such as fatigue or weakness.[1,8]
Hepatotoxicity leading to hepatic failure is
estimated to occur in one in every 24,000 to
34,000 patients taking felbamate. Felbamate
should not be used in patients with a history
of hepatic dysfunction.[1,8]
The need for monitoring drug levels has not
been established. However, baseline laboratory
testing should include a complete blood
count, platelet count and reticulocyte count,
as well as determination of liver enzyme levels.
Hematologic evaluations should be performed
frequently during treatment and after
discontinuation of treatment. Liver enzyme
levels should be determined every one to two
weeks, and felbamate therapy should be
discontinued if the aspartate aminotransferase,
alanine aminotransferase or bilirubin levels
increase above baseline.
Because of serious side effects, felbamate is
not recommended as first-line therapy in the
treatment of seizures. The manufacturer recommends
its use only in patients who do not
adequately respond to alternative therapy and
whose epilepsy is so severe that the substantial
risks of aplastic anemia and hepatic failure are
deemed acceptable.[8] Its use requires that the
physician be thoroughly familiar with the
drug. The manufacturer recommends that
written consent be obtained before initiation
of therapy.
Monotherapy in adults should begin with
1,200 mg of felbamate daily, given in divided
doses every six to eight hours. Daily dosages
should increase by 600 mg every two weeks to
a total daily dosage of 2,400 to 3,600 mg. As
adjunctive therapy, treatment should begin at
1,200 mg daily, given in divided doses every
six to eight hours. If the patient is taking
phenytoin, valproic acid or carbamazepine, a
20 to 35 percent reduction in the dosage of
these drugs is recommended during felbamate
therapy. Levels of antiepileptic drugs should
be followed as the dosage of felbamate is
increased to 2,400 to 3,600 mg daily.
The beginning dosage of felbamate in children
aged two to 14 years with Lennox-Gastaut
syndrome is 15 mg per kg, given in three
to four divided doses. Dosages of other
antiepileptic drugs should be reduced by 20
percent, with further reductions based on side
effects or drug levels. The daily dosage of
felbamate should increase by 15 mg per kg
weekly, to a maximum of 45 mg per kg.[9]
Felbamate is available in 400-mg and 600-mg
tablets, and as a suspension of 600 mg per
5 mL (Table 1). The cost of one month o
treatment at the lowest recommended maintenance
dosage is approximately $52.[6]
Topiramate
Topiramate (Topamax) has been approved
for adjunctive treatment in adults with partial
seizures. It has a novel chemical structure
derived from D-fructose that blocks voltage-sensitive
sodium channels, enhances the activity
of GABA, an inhibitory neurotransmitter,
and blocks the action of glutamate, an excitatory
neurotransmitter. It is also a weak carbonic
anhydrase inhibitor.[11]
Topiramate is well absorbed orally with a
bioavailability of 80 percent. It is less than 20
percent protein bound. When used alone, 20
percent of the drug is metabolized. With
concurrent use of other antiepileptic drugs, 50
percent of the drug is metabolized. Excretion
is primarily renal, with 50 to 80 percent of
each dose excreted unchanged. The half-life is
20 to 30 hours.
A 30 percent and 48 percent median reduction
in seizure frequency occurs at dosages of
200 mg and 400 mg per day, respectively. No
improvement in seizure reduction occurs at
dosages above 400 Mg.[12] The only known
contraindication is hypersensitivity to the drug.
Side effects include dizziness and somnolence
(which are not dose related), ataxia, impaired
concentration, confusion, fatigue, paresthesias,
speech difficulties, diplopia and nausea.[13]
There is an increased risk of nephrolithiasis,
which may be due to carbonic anhydrase inhibition.[14]
Concomitant use of topiramate with
other carbonic anhydrase inhibitors such as
dichlorphenamide (Daranide) or acetazolamide
(Diamox) should be avoided.
Topiramate increases phenytoin concentration
by 25 percent and decreases valproic acid
concentration by 11 percent (Table 2). Topiramate
does not change the concentration of
carbamazepine, phenobarbital or primidone
when coadministered. Concentrations of topiramate
decrease up to 48 percent when
phenytoin is coadministered, up to 40 percent
with coadministration of carbamazepine and
up to 14 percent with valproic acid.
Topiramate is classified as a category C
medication during pregnancy. It is not known
if it is excreted in human breast milk.
Overdoses have been managed to date with
prompt induction of emesis or lavage. Topiramate
is effectively removed by hemodialysis.
The starting dosage is 50 mg per day given
in the evening, increasing by 50 mg per week
until a dosage of 200 mg given twice daily is
reached. It is not necessary to monitor drug
levels. Dosing beyond 400 mg per day does
not increase efficacy. Topiramate can be taken
with food, if desired. Patients with impaired
renal function should use one half the recommended
dosage.
Topiramate is available in 25-mg, 100-mg
and 200-mg coated tablets. The cost of one
month's therapy at 400 mg per day is approximately
$181.[6]
Fosphenytoin
Fosphenytoin (Cerebyx) is a phenytoin
precursor that is rapidly converted after
parenteral administration. It is indicated for
short-term parenteral use when the oral form
is unavailable or less advantageous.[15] In
addition to its use as a short-term substitute for
oral phenytoin, fosphenytoin can be used to
control status epilepticus and to prevent and
control seizures during neurosurgery.
The use of parenteral phenytoin is complicated
by poor solubility, high alkalinity,
hypotension, cardiac arrhythmias and the
potential for soft tissue injury with extravasation.
However, fosphenytoin can be administered
intravenously or intramuscularly with a
low risk of tissue irritation. No significant
electrocardiographic changes have been noted
with either intravenous or intramuscular
administration. Mild decrements in mean systolic
blood pressure have been reported with
intravenous administration.
Therapeutic serum levels of phenytoin are
attained within 10 minutes of infusion of
intravenous fosphenytoin.[16] Peak serum
phenytoin levels are attained 90 minutes after
intramuscular administration. Fosphenytoin
administered intravenously or intramuscularly
is 100 percent bioavailable and is 90 to 95
percent protein bound. A 15-mg dose of
fosphenytoin is equivalent to 1 mg of phenytoin.
Fosphenytoin i6 contraindicated in patients
with hypersensitivity to phenytoin or other
hydantoins, and in patients with sinoatrial
block, second- and third-degree atrioventricular
block and Stokes'-Adams syndrome.[13]
Common adverse effects include pruritus,
nystagmus, dizziness, somnolence, ataxia,
nausea, tinnitus and hypotension. Up to 64
percent of patients experience groin discomfort
on intravenous administration, which
usually dissipates within 60 minutes.
Concomitant use with carbamazepine or
diazepam (Valium) has shown no effect on
fosphenytoin binding. Fosphenytoin binding
did decrease in patients with excessive
concentrations of phenobarbital or valproic acid.
For patients with status epilepticus, 22.5 to
30 mg per kg of fosphenytoin should be
administered intravenously at a rate of 100 to
150 mg per minute. For nonemergent therapy
or to prevent seizures, 15 to 30 mg per kg can
be administered intravenously or intramuscularly
in a loading dose, followed by a daily
maintenance dosage of 6 to 12 mg per kg.
Patients who are already at therapeutic levels
of oral phenytoin can be given fosphenytoin at
1.5 times the daily oral phenytoin dose. The
approximate cost of a 10-mL vial of 750 mg of
fosphenytoin is $54.[6]
The authors thank John Y. Oh, M.D., and Robert L.
Jones, D. Ed., for review of the manuscript.
REFERENCES
[1.] Dichter MA, Brodie MJ. New antiepileptic drugs. N
Engl J Med 1996;334:1583-90.
[2.] Ramsay RE. Advances in the pharmacotherapy of
epilepsy. Epilepsia 1993;34(Suppl 5):S9-16.
[3.] Gabapentin. Package insert. Morris Plains, N.J.:
Parke-Davis, December 1994.
[4.] Laxer KD. Guidelines for treating epilepsy in the
age of felbamate, vigabatrin, lamotrigine, and
gabapentin. West J Med 1994; 161:309-14.
[5.] Rosner H, Rubin L, Kestenbaum A. Gabapentin
adjunctive therapy in neuropathic pain states. Clin
J Pain 1996;12:56-8.
[6.] Red book. Montvale, N.J.: Medical Economics
Data, 1997.
[7.] Lamotrigine. Package insert. Research Triangle
Park, N.C.: Glaxo Wellcome Inc., March 1997.
[8.] Palmer KJ, McTavish D. Felbamate. A review of its
pharmacodynamic and phamacokinetic properties,
and therapeutic efficacy in epilepsy. Drugs 1993;
45(6):1041-65.
[9.] Jensen PK. Felbamate in the treatment of refractory
partial-onset seizures. Epilepsia 1993;34(Suppl
7):S25-9.
[10.] Felbamate. Package insert. Cranbury, N.J.: Wallace
Laboratories, November 1995.
[11.] Medical Science Bulletin 1997;20(236):1.
[12.] Faught E, Wilder BJ, Ramsay RE, Reife RA, Kramer
LID, Pledger GW, et al. Topiramate placebo-controlled
dose-ranging trial in refractory partial epilepsy using
200-, 400- and 600-mg daily dosages. Neurology
1996;46:1684-90.
[13.] Olin B, ed. Drug facts and comparisions. St. Louis:
Facts and Comparisons, Inc., 1997:283u-y.
[14.] Shorvon SD. Safety of topiramate: adverse events
and relationships to dosing. Epilepsia 1996;37
(Suppl 2):18-22.
[15.] Fosphenytoin. Package insert. Morris Plains, N.J.:
Parke-Davis, 1996.
[16.] Allen FH Jr, LeGarda S, et al. Safety, tolerance, and
pharmacokinetics of intravenous fosphenytoin
(Cerebyx) in status epilepticus [Abstract]. Epilepsia
1995;36(Suppl 4):90.
WILLIAM J. CURRY, M.D., is co-medical director of Pennsylvania State Family
Health, Middletown, and assistant professor in the Department of Family and
Community Medicine at the Milton S. Hershey Medical Center, Hershey, Pa. He
received his medical degree from Pennsylvania State University College of
Medicine, Hershey, and completed a residency in family practice at the U.S.
Air Force Regional Hospital Eglin, Eglin Air Force Base, Fla.
DAVID L. KULLING, M.D., is assistant professor in the Department of Family
and Community Medicine and the Department of Orthopaedics and Rehabilitation
at the Milton S. Hershey Medical Center. He graduated from the Muntch
University of Technology and completed a residency in family practice at the
Allegheny Family Physicians Residency Program of the Altoona (Pa.) Hospital.
He also completed a primary care sports medicine fellowship at the University
of Michigan Medical School, Ann Arbor. He holds certificates of added
qualification in geriatric medicine and sports medicine.
Address correspondence to David L. Kulling, M.D., Pennsylvania State
University, Milton S. Hershey Medical Center Department of Family and
Community Medicine, PO. Box 850, Hershey PA 17033-0850. Reprints are not
available from the authors.
Richard W. Sloan,
M.D., R.PH.,
coordinator of this
series, is chairman
and residency
program director
of the Department
of Family Medicine at
York (Pa.) Hospital
and clinical associate
professor in family
and community
medicine at the
Milton S. Hershey
Medical Center,
Pennsylvania State
University, Hershey, Pa.
COPYRIGHT 1998 American Academy of Family Physicians
COPYRIGHT 2000 Gale Group