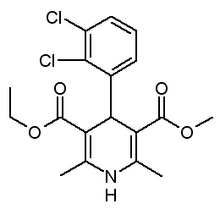
United Research Laboratories/Mutual Pharmaceutical company reported that the FDA has accepted for filing Mutual Pharmaceutical company's ANDA for felodipine extended-release tablets, reference drug Plendil extended-release tablets (registered trademark of AstraZeneca, LP).
URL/Mutual believes its filing is the first Abbreviated New Drug Application for felodipine extended-release tablets. The company's filing includes a Paragraph IV Certification, which asserts that the Company's formulation does not infringe on listed patents on the drug. As such, upon final FDA approval, the company expects a period of exclusivity for its generic Plentil lasting not less than six months. Because of the challenging nature of the successful formulation of the drug, URL/Mutual also expects its exclusivity may extend beyond the six-month period.
The U.S. market for Plendil extended-release tablets is in excess of $150 million, growing at a rate of 24 percent annually, the company stated.
COPYRIGHT 2000 Lebhar-Friedman, Inc.
COPYRIGHT 2000 Gale Group