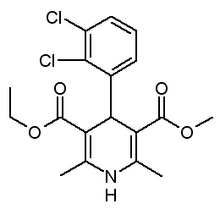
Pulmonary hypertension in chronic obstructive pulmonary disease (COPD) is associated with a poor prognosis. Reduction of pulmonary artery pressure in COPD by prolonged oxygen treatment has been shown to be associated with increased survival. In an attempt to find a suitable pharmacologic method of reducing pulmonary artery pressure and pulmonary vascular resistance in COPD, we enrolled 13 stable pulmonary-hypertensive, hypoxemic COPD patients in a study to test the effects of felodipine, a relatively new, vascular-selective calcium antagonist. Doppler echocardiography was used to estimate pulmonary artery pressure and cardiac output before treatment, 2, 7, and 12 weeks during felodipine treatment (10 to 20 mg/d), and after a 1-week placebo washout period. Measurements of lung function, arterial blood gases, and exercise capacity during an incremental bicycle ergometer test were also performed at intervals during the study period. Three patients withdrew from the study and of the remaining 10, 8 had some side effects of medication (peripheral edema or headache) that improved either spontaneously or following a reduction in drug dose. In the 10 patients who completed the study (8 male; mean age, 67 years), felodipine resulted in significant reductions in mean pulmonary artery pressure (22 percent) and total pulmonary (vascular) resistance (30 percent) and increases in cardiac output (15 percent) and stroke volume (13 percent) compared with baseline measurements and those taken after placebo washout. These effects were sustained over the 12 weeks of felodipine treatment. There was no adverse effect of felodipine treatment on pulmonary gas exchange at rest or during exercise and no change in lung function or exercise capacity. We conclude that in pulmonary hypertensive, hypoxemic COPD patients, felodipine substantially improves pulmonary hemodynamics.
Pulmonary hypertension is a frequent complication of chronic obstructive pulmonary disease[1,2] (COPD) and is associated with reduced survival in this disorder.[3,4] Hypoxic pulmonary vasoconstriction appears to be the most important cause of pulmonary hypertension in COPD.[5,6] However, a persistently elevated pulmonary artery pressure (PAP) causes secondary hyperplasia and hypertrophy of the vessel intima and media (vascular "remodeling") which further decreases vascular caliber and distensibility and increase arterial pressure.[7,8]
The most effective treatment found thus far for pulmonary hypertension in COPD is prolonged oxygen administration.[9,10] This decreases PAP and increases longevity. However, there are practical limitations to oxygen therapy and most patients are prescribed oxygen for limited periods each day (typically 12 to 15 h). Continuous pharmacologic inhibition of hypoxic pulmonary vasoconstriction has been proposed as a cost-effective adjunctive therapy.[11-13] While some of the studies of vasodilator therapy in COPD report disappointing results,[14,15] recent evidence suggests that some of the newer, vascular-selective calcium antagonists may be more effective.[16-20] Felodipine is a calcium antagonist with a prolonged duration of action and high vascular selectivity.[21,22] A recent study indicated that it might have a beneficial effect on pulmonary hemodynamics in COPD.[23]
In the present study, we report the effects of 12 weeks of felodipine treatment in pulmonary hypertensive, hypoxemic COPD patients. Pulmonary artery pressure and cardiac output (CO) were measured repeatedly over the study period by pulsed Doppler echocardiography. The results showed a sustained beneficial effect on pulmonary hemodynamics during the treatment period.
METHODS
The research protocols used in this study were approved by the Ethics Committees of the Royal Adelaide Hospital and the Repatriation General Hospital, Daw Park, and all patients gave written informed consent.
Study Design
Clinically stable patients with COPD and secondary pulmonary hypertension were studied before, during 12 weeks of felodipine treatment, and 1 week after placebo washout of felodipine. Baseline measurements of lung function, exercise capacity, and pulmonary hemodynamics were made. This was followed by a 14-day titration of felodipine. The starting dose was 2.5 mg (bid) and this was increased every 5 days, first to 5.0 mg bid and then 10 mg bid. The protocol required that the final dose of felodipine be reached 4 days before the end of the titration period. Felodipine therapy was continued for 10 more weeks after which a placebo was administered for 1 additional week. Felodipine and placebo were administered single blind. Provision was made for the felodipine dose to be reduced to the next lowest dose in the event of inconvenient or uncomfortable side effects. The baseline (week 0) measurements listed above were repeated after drug titration (week 2), 5 and 10 weeks later while receiving maximum tolerated treatment (weeks 7 and 12), and after 1 week of drug washout with placebo (week 13).
Patient Selection
Patients were recruited from the outpatient departments of the Royal Adelaide Hospital and the Repatriation General Hospital, Daw Park, South Australia. For entry they were required to have a diagnosis of chronic bronchitis and/or emphysema secondary to cigarette smoking, to have been stable with no clinical exacerbation in the preceding 3 months, and to have had stable hypoxemia (Pa[O.sub.2] <70 mm Hg) over the same period. Evidence of chronic airflow limitation ([FEV.sub.1] <70 percent predicted and [FEV.sub.1]/FVC ratio <70 percent) was required and each of the patients had to have an analyzable Doppler signal (see below) with evidence of pulmonary hypertension (Doppler-estimated supine systolic pulmonary artery pressure >30 mm Hg and/or mean PAP >20 mm Hg).
Patients were excluded if they had any of the following: (1) history of asthma or >20 percent increase in [FEV.sub.1] following bronchodilator; (2) history of primary cardiac disease or resting or exercise ECG abnormality indicating myocardial ischemia; (3) use of [beta]-blocking drugs, antiarrhythmic agents, nitrates, or other vasodilators through the study period; (4) bundle branch block or ventricular outflow obstruction; (5) hemoglobin <12 g/100 ml; (6) any severe concomitant disease that could interfere with survival or well-being (eg, renal failure, unstable diabetes, cancer) and; (7) any known vascular, orthopedic, or neurologic problems of the lower extremities that might interfere with bicycle exercise. Patients older than 70 years were excluded. All regular medications and oxygen therapy (prescribed in four patients) were kept constant throughout the study period.
Twenty-two patients met the lung function and clinical entry criteria and were selected for Doppler screening. Fifteen patients had an analyzable Doppler signal for hemodynamic calculations and 13 of them met the criteria for pulmonary hypertension. Of these, three patients (two male and one female) were subsequently withdrawn because of drug side effects. The baseline characteristics of the ten patients (eight male and two female) who completed the study protocol are shown in Table 1.
Felodipine Compliance, Side Effects, and Plasma Concentrations
Patients were encouraged to report any side effects immediately. At 0, 2, 7, 12, and 13 weeks, patients were questioned about any adverse events. Compliance with felodipine or placebo treatment was assessed by counting residual tablets and venous blood was taken on first presenting to the laboratory (2 h after the morning dose of felodipine or placebo) for plasma felodipine assay. Felodipine concentrations were determined in heparinized plasma samples by high-performance liquid chromatography with fluorescence detection. Plasma samples (2.0 ml), with internal standard (a felodipine analogue, 154/87, Astra, Hassle, Sweden) added, were extracted with dichloromethane (5.0 ml). This extract was dried under nitrogen at 40[degrees]C into octanol (10 [mu]l) and subsequently diluted into n-hexane (100 [mu]l). The normal-phase chromatography included a mobile-phase of n-hexane : ethanol : ammonia solution (91.2 : 7 : 1.8; v:v:v) pumped at 2.0 ml/min through a 5-[mu]m silica column (440 x 4.6 mm, Brownlee Laboratories, part number SS-224). Column eluates were detected fluorimetrically (Perkin Elmer, model LS-1) with excitation and emission wavelengths of 366 and 430 nm, respectively. The retention times for felodipine and 154/87 were 7.5 and 9.5 min, respectively, and the detection limit was 2.5 nmol/L.
Assessment of Cardiopulmonary Function
At weeks 0, 2, 7, 12, and 13 measurements were made of forced expiratory lung volumes (Ohio spirometer), resting supine arterial blood gases (Radiometer ABL 3 blood gas analyzer, Copenhagen, Denmark), lung carbon monoxide transfer factor (Jaeger MasterLab system v.2.02), and pulsed Doppler echocardiography[24-27] (Hewlett Packard 77020A Ultrasound imaging system, Andover, Mass). Doppler echocardiography (see companion article) was used to estimate systolic and mean [PAP.sup.25] and [CO.sup.26] and from these parameters total pulmonary resistance (TPR) was estimated by dividing mean PAP by CO. Cardiac index (CI) was calculated by dividing CO by body surface area (BSA). The BSA was calculated from the following formula: BSA ([m.sup.2]) = weight ([kg.sup.0.425] x height ([cm.sup.0.725]) x 0.007184. Hemoglobin level (Hb) and oxyhemoglobin saturation ([SaO.sub.2]) were measured by CO-oximeter (OSM2 Hemoximeter, Radiometer, Copenhagen) and arterial oxygen content ([CaO.sub.2]) was calculated from the following formula: [CaO.sub.2] = (1,34 x Hb) x [SaO.sub.2] + 0,003 x [PaO.sub.2]. Oxygen delivery was derived by multipling [CaO.sub.2] by CI.
At weeks 0, 2, 12, and 13 incremental bicycle ergometer exercise testing was performed. The stage 1 protocol of Jones and [Campbell.sup.28] was followed. The tests were performed between 4 and 8 h after the morning felodipine dose and after pulmonary function testing and the Doppler measurements. Patients rode an electronically braked bicycle ergometer with work rate increased in 50 kpm (8 W) increments each minute from a starting level of 50 kpm. Patients were asked to exercise until they could not continue. Pulse rate, respiratory rate, and breath-by-breath expired ventilation, and [VO.sub.2] and [Vco.sub.2] were computed and averaged for each minute. Arterial oxygen saturation was measured continuously by pulse ear oximetry (Biox Ohmeda 3700, Boulder, Colo). [TABULAR DATA OMITTED]
Statistical Analysis
The data were analyzed using statistical software (Statview II, Abacus Concepts, Berkeley, Calif) on a microcomputer (Apple Macintosh SE30). Data were first subjected to a repeated measures analysis of variance tests. If the F statistic for a particular parameter reached statistical significance (p<0.05), then pairwise comparisons were made between mean values obtained at baseline or following placebo washout with those obtained during treatment using the Fisher's "protected" least significant difference method.
RESULTS
Felodipine Concentrations, Complications, and Side Effects
Three patients withdrew from the study, two because of marked edema of the lower extremities not responding to a decreased dose of felodipine (ie, from 10 to 5 mg bid) and one because of dizziness during the tests.
In the ten patients who completed the study, the most common side effect was ankle edema (seven patients). It was described as severe by five patients (all responded well to down titration of felodipine dose from 10 to 5 mg bid), and moderate or mild by one patient each (no dose change required). The next most common side effect was headache (six patients). It was described as severe by one patient, moderate by two patients, and mild by three patients. The patient with severe headache responded well to a down titration of felodipine dose; the patients with moderate or mild headache needed no alteration in drug dose. Dizziness was experienced by two patients (reported as moderate in one, and mild in the other). Facial flushing was reported in two patients (both mild). One patient reported short-term mild palpitations. Most of the side effects occurred at the maximum dose of felodipine (10 mg bid) and responded to a reduction in dose. Felodipine plasma concentrations are shown in Figure 1.
Respiratory Function
Lung function and arterial blood gas parameters are presented in Table 2. There were no statistically significant differences in any of the parameters over the study period. [TABULAR DATA OMITTED]
Hemodynamics
The effect of felodipine on a number of important hemodynamic parameters is shown in Table 3. There were significant falls of 22 percent and 32 percent in PAP and TPR, respectively, during felodipine treatment. There was also a small fall (7 percent) in mean systemic arterial pressure. These changes were accompanied by increases in stroke volume (13 percent), CO (15 percent), and oxygen delivery (22 percent). All these changes were sustained during the 12-week treatment period despite a reduction in plasma drug concentrations. Hemodynamic parameters returned toward baseline levels a week after cessation of felodipine treatment.
Exercise Capacity
Exercise parameters are presented in Table 4. There were no statistically significant differences in any of the parameters during felodipine treatment. The [O.sub.2] consumption and [CO.sub.2] production data are not presented because errors in the computer-assisted breath-by-breath calculations led to unreliable results in several of the patients.
DISCUSSION
Pulmonary hypertension appears to be an important, possibly independent, risk factor for mortality in COPD.[4,29] In assessing the effects of long-term domiciliary oxygen therapy in COPD in an earlier study, it was found that patients who demonstrated the greatest fall in PAP after 6 months of treatment had the longest survival.[30] It is not surprising, therefore, that there has been an extensive and prolonged search for an effective pharmacologic method of reducing PAP and pulmonary vascular resistance (PVR) in this disease. The topic has been the subject of several comprehensive review.[11-13,29] Unfortunately, the results of investigations have thus far been somewhat disappointing and vasodilator agents do not yet have a place in the routine treatment of patients with pulmonary hypertensive COPD.
The chief problems with vasodilator therapy in COPD have been that (1) the agents have had minimal effects on the pulmonary circulation, (2) tachyphylaxis develops over a short time, or (3) vasodilator therapy produces a worsening of pulmonary gas exchange and ventilation-perfusion matching secondary to release of hypoxic vasoconstriction in poorly ventilated lung regions. The inability to find a suitable drug has impeded progress toward large-scale clinical trials of vasodilators in COPD, and to date and to our knowledge, no properly controlled study has been performed. [TABULAR DATA OMITTED]
Calcium channel blockers are a group of drugs with potential therapeutic value in COPD. They have been shown to prevent and reverse hypoxia-induced pulmonary hypertension in animals[31,32] and to improve pulmonary hemodynamics in patients with primary pulmonary hypertension.[33] While verapamil and diltiazem failed to lower resting or exercise PAP or PVR in patients with hypoxic pulmonary hypertension when given with supplemental oxygen,[34,35] nifedipine, nitrendipine, and felodipine have all been shown to acutely lower PAP and PVR.[17-20] A recent study[23] showed a sustained reduction in PVR and an increase in CO during 12 weeks of felodipine treatment in 8 COPD patients, and another study[19] using nitrendipine showed similar improvements during 6 weeks of treatment. In contrast, Agostoni et al[15] found that the initial positive effects of nifedipine were not sustained after 8 weeks of treatment.
In the present study, using the relatively new vasoselective calcium antagonist, felodipine, we have been able to show falls in mean PAP and total pulmonary resistance of more than 20 percent and 30 percent, respectively. This effect was sustained despite a reduction in mean drug dose and plasma drug concentration over the 3-month treatment period (Fig 2). There was a concomitant increase in cardiac output and calculated oxygen delivery at rest (Fig 3). Importantly, these changes occurred without any adverse effect on pulmonary gas exchange at rest or during incremental exercise. The observed improvements in pulmonary hemodynamics during felodipine treatment were much greater than those found in a previous study[23] following the short-term administration of supplemental oxygen to COPD patients.
Doppler echocardiography is a recent important advance that has opened the possibility for more extensive investigation of pulmonary hemodynamics in COPD. In this study, we performed six measurements, including the initial screening study over a 4-month period on each patient. These repeated measurements increased the statistical power of the study and confidence in the results and it would not have been possible, we believe, to ethically obtain similar data by right heart catheterization. We have shown in another study[27] close agreement (r = 0.97) between Doppler and catheter-derived measurements of both PAP and CO. Doppler echocardiography in COPD patients, however, has limitations. The chief one is that suitable images cannot be obtained in all patients. Seventy percent of COPD patients who were screened for the present study using the technique of Morera et al[25] had suitable images.[27] Another limitation at the time this study was conducted was that we could not estimate left atrial pressure by Doppler techniques. Therefore, we used TPR (ie, mean PAP divided by CO) rather than PVR ((mean PAP - pulmonary capillary wedge pressure)/CO) to assess the vasodilator effect of felodipine on the pulmonary circulation. We estimated from the change in TPR that felodipine reduced resistance within the pulmonary circulation by approximately one third. However, this conclusion is predicated on the assumption that felodipine did not change left atrial pressure. Since our patients did not have left ventricular or mitral valve disease, we do not believe felodipine would have changed left atrial pressure appreciably. However, in the unlikely event that it did reduce left atrial pressure, our conclusion that felodipine produced a significant reduction in PVR would not have been altered. Our results indicate that had felodipine reduced left atrial pressure by 5 mm Hg, the resistance of the pulmonary circulation would still have been decreased by approximately 25 percent.
We observed a 15 percent increase in CO following felodipine. The question arises therefore as to the possible contribution of this increase in pulmonary blood flow itself on the observed decrease in TPR. It is known that increase in CO may lead to a distention and recruitment of pulmonary blood vessels, thereby lowering PVR. We cannot exclude this as a possible mechanism for the changes we observed; however, we consider its effect on the observed decrease in TPR was probably minor. Under physiologic conditions, an increase in CO results in no change or a slight increase in PAP, while in COPD (eg, during exercise), PAP often rises sharply because of the relative inability to distend and recruit pulmonary vessels. In our study, PAP decreased (20 percent) rather than increased in the face of the increased CO, indicating that there was significant direct pharmacologic vasodilation of the pulmonary circulation by felodipine.
Only one other group has, to our knowledge, studied the effects of felodipine on pulmonary hemodynamics in COPD patients. Bratel and associates[23] recently reported the immediate and long-term follow-up results of felodipine in eight patients with severe COPD. They performed right heart catheterizations and found after a mean of 14 weeks of treatment a 16 percent reduction in PVR, a 20 percent increase in CO, but no change in mean PAP. Our results are similar, but the changes we observed in pulmonary hemodynamics were more marked. We found a 22 percent fall in mean PAP, a 15 percent increase in CO, a fall in TPR of 32 percent, and a fall in PVR that we estimated to be at least 25 percent. The reasons for our different results probably relate to several differences in study design: first, an entry criterion of our study was that patients have demonstrable pulmonary hypertension (mean PAP >20 mm Hg), whereas this was not the case in the study of Bratels et al. Although our patients appeared to have less severe lung function disturbance and hypoxemia than those in the study by Bratel et al, pulmonary hemodynamic measurements suggest that they had more pulmonary hypertension. Second, Bratel et al assessed the effects of felodipine while patients breathed supplemental oxygen to reverse hypoxemia (and presumably also hypoxic pulmonary vasoconstriction), whereas we studied the effects of felodipine during room air breathing. Third, we used a higher maintenance dose of felodipine (10 to 20 mg/d) than Bratel et al (7.5 to 15 mg/d). An earlier study by the same group[36] showed inconsistent effects of 3 to 5 months of felodipine treatment on pulmonary haemodynamics in COPD patients. However, the patients in that study had less pulmonary hypertension (mean PAP, 22 mm Hg) compared with that in their subsequent study[23] (mean PAP, 29 mm Hg) and patients in our study (mean PAP, 33 mm Hg).
The present study showed a trend toward increased exercise duration and work capacity during felodipine treatment, but this did not reach statistical significance. This result is similar to that reported by Bratel et al[23,36] in two other studies in felodipine and exercise capacity in COPD, and is consistent with the equivocal findings on vasodilators and exercise in COPD generally.[29] This is perhaps not surprising since the principle limitation to exercise in COPD patients appears to be inadequate ventilatory reserves rather than a cardiac or circulatory limitation. However, in COPD patients with severe pulmonary hypertension, such as those enrolled in the present study, cardiac limitations may begin to become apparent. It is interesting to note that at the maximum work load achieved by our patients, ventilation for the group was within 30 percent of predicted maximum voluntary ventilation, indicating ventilatory reserves had been exhausted,[37] but also the maximum heart rate was 90 percent of that predicted for the mean age of the patients studied (predicted maximum heart rate: 210--age [years] = 210 - 67 = 143 bpm), suggesting that patients were also close to their maximum exercise cardiac function.
A notable feature of this study was the high incidence of drug side effects. Pedal edema and dizziness necessitated the withdrawal from the study of 3 of 13 patients and of the remainder, 80 percent reported either ankle swelling or headache. This incidence of side effects is similar to that reported by Bratel and associates.[23] In each of the patients in our study, symptoms subsided with time or a reduction in the drug dose. All of these side effects are well known with other dihydropyridine calcium antagonists and are the result of vasodilation of systemic arterioles. Since the hemodynamic effects of felodipine treatment persisted at lower plasma drug concentrations during the latter part of the treatment period, it is reasonable to believe that a lower daily dose of felodipine, perhaps using the newer sustained-release form of the drug, which produces lower peak plasma felodipine concentrations, could be used in future studies of pulmonary hypertensive COPD patients. A lower dose may be equally effective and better tolerated.
In summary, we have shown marked improvements in pulmonary hemodynamics in pulmonary hypertensive hypoxemic COPD patients during 12 weeks of felodipine administration. The incidence of side effects from felodipine was relatively high in this study and this may constitute a significant impediment to the successful conclusion of large-scale clinical trials. However, side effects appeared to be dose-related and our data suggest that the maintenance dose of felodipine needed to lower PAP and PVR in COPD may well be less than we initially chose.
ACKNOWLEDGMENTS: We wish to acknowledge the financial support of Astra Pharmaceuticals; Mr. Michael Clark (Rehabilitation Studies, Repatriation General Hospital, Daw Park) for statistical advice; Prof Derek Frewin for his encouragement and advice; and our clinical colleagues at the Royal Adelaide and Repatriation Hospitals who agreed to the participation of their patients in this study.
References
[1] Fishman AP. Chronic cor pulmonale. Am Rev Respir Dis 1976; 114:775-94
[2] Rounds S, Hill NS. Pulmonary hypertensive diseases. Chest 1984; 85:397-405
[3] Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener C. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med 1972; 272:912-18
[4] Weitzenblum E, Hirth C, Duclone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981; 36:752-58
[5] Staub N. Site of hypoxic pulmonary vasoconstriction. Chest 1985; 88:240-55
[6] Wright JL, Lawson L, Pare PD, Hooper RO, Peretz DI, Nelems JMB, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. Am Rev Respir Dis 1983; 126:702-07
[7] Reid LM. Structure and function in pulmonary hypertension (new perceptions). Chest 1986; 89:279-88
[8] Magee F, Wright JL, Wiggs BR, Pare PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive lung disease. Thorax 1988; 43:183-89
[9] Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. Ann Intern Med 1980; 93:391-98
[10] Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1:681-86
[11] Hyman AL, Hadowitz PJ. Vasodilator therapy for pulmonary hypertensive disorders. Chest 1984; 85:145-47
[12] White KF, Flenley DC. Can pulmonary vasodilators improve survival in cor pulmonale due to hypoxic chronic bronchitis and emphysema? Thorax 1988; 43:1-8
[13] Morley T, Zappasodi S, Belli A, Guidice J. Pulmonary vasodilator therapy for chronic obstructive pulmonary disease and cor pulmonale. Chest 1987; 92:71-6
[14] Vik-Mo H, Walde N, Jentoft H, Halvorsen FJ. Improved haemodynamics but reduced arterial blood oxygenation, at rest and during exercise after long-term oral prazosin therapy in chronic cor pulmonale. Eur Heart J 1985; 6:1047-53
[15] Agostoni P, Doria E, Galli C, Tamborini G, Guazzi MD. Nifedipine reduces pulmonary pressure and vasodilator tone during short-but not long-term treatment of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 139:120-25
[16] Kennedy T, Michael J, Huang C, Kallman CH, Zahka K, Schlott W, et al. Nifedipine inhibits hypoxic pulmonary vasoconstriction during rest and exercise in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1984; 129:544-51
[17] Saadjian A, Philip-Joet F, Arnaud A. Hemodynamic and oxygen delivery: responses to nifedipine in pulmonary hypertension secondary to chronic obstructive lung diseases. Cardiology 1987; 74:196-204
[18] Muramoto A, Caldwell J, Albert RK, Lakshminarayan S, Butler J. Nifedipine dilates the pulmonary vasculature without producing symptomatic systemic hypotension in upright resting and exercise patients with pulmonary hypertension secondary to chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 132:963-66
[19] Rubin LJ, Moser K. Long-term effects of nitrendipine on hemodynamics and oxygen transport in patients with cor pulmonale. Chest 1986; 89:141-45
[20] Bratel T, Hedenstierna G, Lindquist H, Nyquist O, Ripe E. Cardiac function and central hemodynamics in severe chronic obstructive lung disease: acute and long-term effects of felodipine. Eur Respir J 1988; 1:262-68
[21] LJung B. Vascular selectivity of felodipine. Drugs 1985; 29(suppl 2):46-58
[22] Ljung B, Nordlander M. Pharmacodynamic properties of felodipine. Drugs 1987; 29(suppl 3):7-15
[23] Bratel T, Hedenstierna G, Nyquist O, Ripe E. The use of vasodilator, felodipine, as an adjuvant to long-term oxygen treatment in COLD patients. Eur J Respir Dis 1990; 3:46-54
[24] Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in two-dimensional echocardiography. Circulation 1980; 62:212-17
[25] Morera J, Hoadley SD, Roland JM, Pasipoularides A, Darragh R, Gaitan G, et al. Estimation of the ratio of pulmonary to systemic pressures by pulsed-wave Doppler echocardiography for assessment of pulmonary arterial pressures. Am J Cardiol 1989; 63:862-66
[26] Spodick DH, Koito H. Nongeometric Doppler stroke volume determination. Am J Cardiol 1989; 63:883-84
[27] Sajkov D, McEvoy RD, Cowie RJ, Bradley JA, Mahar L. Validation of new pulsed Doppler echocardiographic techniques for assessment of pulmonary hemodynamics. Chest (in press)
[28] Jones NL, Campbell EJM. Clinical exercise testing. Philadelphia: WB Saunders Co, 1982
[29] Klinger JR, Hills NS. Right ventricular dysfunction in chronic obstructive pulmonary disease: evaluation and management. Chest 1991; 99:715-23
[30] Timms RM, Khaja FU, Williams GW, the Nocturnal Oxygen Therapy Trial Group. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med 1985; 102:29-36
[31] Stanbrook HS, Morris KG, McMurtry IF. Prevention and reversal of hypoxic pulmonary hypertension by calcium antagonists. Am Rev Respir Dis 1984; 130:81-5
[32] Michael JR, Kennedy TP, Buescher P, Farrukh IM, Lodato RT, Rock PC, et al. The effect of treatment with nitrendipine and other calcium channel blockers on the physiologic and pathologic changes caused by hypoxia in rats. J Cardiovasc Pharmacol 1987; 9(suppl 4):S61-S65
[33] Rich E, Kaufmann E. High dose titration of calcium channel blocking agents for primary pulmonary hypertension: guidelines for short-term drug testing. J Am Coll Cardiol 1991; 18:1323-27
[34] Brown SE, Linden GS, King RR, Blair GP, Stanbury DW, Light RW. Effects of verapamil on pulmonary hemodynamics during hypoxaemia, at rest, and during exercise in patients with chronic obstructive pulmonary disease. Thorax 1983; 38:840-44
[35] Clozel JP, Delorme N, Battistella P, Breda JL, Polu JM. Hemodynamic effects on intravenous diltiazem in hypoxic pulmonary hypertension. Chest 1987; 91:171-75
[36] Bratel T, Hedenstierna G, Nyquist O, Ripe E. Long-term treatment with a new calcium antagonist, felodipine, in chronic obstructive lung disease. Eur Respir J 1986; 68:351-61
[37] Eschenbacher WL, Mannina A. An algorithm for the interpretation of cardiopulmonary exercise tests. Chest 1990; 97:263-67
COPYRIGHT 1993 American College of Chest Physicians
COPYRIGHT 2004 Gale Group