THIS ALLERGY MEDICATION MAY EXACERBATE CARDIAC PROBLEMS
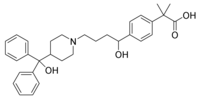
A 67-YEAR-OLD MAN who'd been taking 180 mg of fexofenadine (Allegra) daily for 2 months developed syncope and collapsed. On admission to the hospital, his ECG displayed an abnormally prolonged QT interval. When the fexofenadine was discontinued, his QT interval shortened. It became prolonged again when the drug was restarted on the sixth day. (Why the drug was restarted in this case isn't clear.)
On the 11th day, the patient developed polymorphic ventricular tachycardia (torsades de pointes), which progressed to ventricular fibrillation. He was successfully defibrillated, and the fexofenadine was discontinued again. His QT interval then shortened but remained longer than normal even when he wasn't taking any medication.
What went wrong?
The patient had been taking fexofenadine, a histamine, receptor blocker, for unexplained itching and was on no other medications. However, he was taking a dose 50% greater than the recommended regimen of 60 mg b.i.d. Possibly a subclinical cardiac abnormality-a prolonged QT interval-made him susceptible to the effects of fexofenadine.
What precautions can you take?
When monitoring a patient's drug therapy, be alert for unusual adverse drug reactions, even if they're not specified in the drug labeling. Rare adverse reactions may not become apparent for years after a drug hits the market. Problems with terfenadine, which triggered arrhythmias in some patients, weren't recognized for almost 10 years after that drug went into widespread use. (It's since been withdrawn from the market. Interestingly, fexofenadine was developed in response to problems with terfenadine.)
Follow your facility's policy to report any suspected problems with fexofenadine or any other relatively new agent.
Source: "QT Lengthening and Life-Threatening Atrhythmias Associated with Fexofenadine," Lancet, Y. Pinto, et at., June 12,1999.
Dr. Shuster is clinical associate professor, Temple University and clinical pharmacist, Medical Colles of adverse of Pennsylvania Hospital, both in Philadelphia, Pa. The Institute r a examples of adverse drug reactions to: Joel Street Rd., Warminster, PharmD The Institute for Safe If we publish your item Practices, we'll pay Street Rd., Warminster, PA 18974. Fax: 215-956-9266. If we publish your item (anonymously), we'll pay you $25.
Copyright Springhouse Corporation Sep 1999
Provided by ProQuest Information and Learning Company. All rights Reserved