The Photochemistry of Flutamide and its Inclusion Complex with P-Cyclodextrin. Dramatic Effect of the Microenvironment on the Nature and on the Efficiency of the Photodegradation Pathways
ABSTRACT
The photochemistry of the anticancer drug flutamide (FM), 2-methyl-N-[4-nitro-3-(trifluoromethylpropanamide, in homogeneous media and in the P-cyclodextrin (PCD) cavity has been investigated. The photoreactivity of the free molecule has been rationalized on the basis of an intramolecular nitro to nitrite rearrangement followed by cleavage of the nitrite intermediate. The twisted geometry of the nitro group with respect to the aromatic plane plays a key role in triggering such a photoprocess. Incorporation of FM in the I-CD cavity leads to dramatic effects on both the efficiency and the nature of the photochemical deactivation pathways of the guest molecule. A 20-fold increase in the FM photodecomposition quantum yield and the formation of photoproducts originated by both reduction of the vitro group and cleavage of the amide bond were observed in the presence of the macrocycle. Such a behavior cannot be attributed exclusively to the micropolarity of PCD and/or to its role as a reactant The induced circular dichroism spectra and the nature of the photoproducts formed in these experimental conditions provide indications that the photoreactivity in the P-CD microenvironment could likely be mediated by structural changes of FM upon complexation.
INTRODUCTION
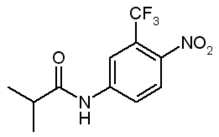
Flutamide (FM)t, 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide, is a nonsteroidal antiandrogen drug that blocks androgen receptor sites; it is commonly used in advanced prostate cancer (1-3). Recent reports have shown the capability of FM to induce in vivo phototoxic effects upon UVA light excitation (4-9). Despite these welldocumented cases of liver phototoxicity, the complex issue concerning the mechanism of FM photosensitization is still unclear. This is probably due to the lack of data concerning the photochemical behavior of FM. To the best of our knowledge, no reports on the photochemistry of this drug appear in the literature up to now (10). It is generally accepted that shedding light on drug photochemical behavior is a prerequisite to understand the molecular basis of the drug-photoinduced biological damage. In this connection, a sequential approach consisting in extending the knowledge concerning the photobehavior in homogeneous media to biological mimicking systems of increasing complexity, represents an adequate strategy for a more appropriate correlation between phototoxicity and photochemical properties. Actually, properties such as hydrophobicity, geometry, size, shape and charge often lead to the formation of supramolecular aggregates between photosensitizer and biomolecule. These aggregates can display different photobehavior when compared to that of the "free molecule." Among the several biological mimetic systems, cyclodextrins (CD) represent one of the most simple ones. Photochemical investigation in such a kind of microenvironment is an extremely active area of research for both fundamental studies and for practical purposes (11). The most important property of these molecules is the ability to form host-guest inclusion complexes with a great variety of organic and inorganic substrates (1218). A number of factors influence complexation. Among them, the "goodness of fit" between the host and the guest and the hydrophobic effects are probably the most important. The weak binding forces responsible for association to the constrained microenvironment of CD provide a useful model to mimic the interactions of drugs with hydrophobic pockets of biological substrates. As already reported for a large variety of systems, CD have extensively proven their potential as media for controlling chemical and photochemical reactions (19-21). The nature of the lowest excited states of the guest molecule, the efficiency of its deactivation pathways, the fate of the reaction intermediates and the opening of new photoreactive channels, are some of the parameters that may be modulated by the CD microenvironment.
Due to their molecular structure, many drugs are suitable guests for CD receptors. In this regard, photophysics and photochemistry of drug-CD inclusion complexes have received considerable attention, especially in the last few years (22-26). The use of CD as a drug complexing agent can represent in fact not only a suitable biological mimicking environment but also a valid tool to minimize the drug-photoinduced biological damage as well as to increase the drug photostability (24,27-29).
This report centers on the photoreactivity of FM both in the absence and in the presence of [-CD in order to contribute to the general picture of drug photochemistry. Beyond this strict photochemical point of view, in light of the reasons described above, the present investigation may represent a step forward in the understanding of the phototoxic effects induced by FM. Finally, it is worth to mention that, due to the poor water solubility of FM, efforts have been made to improve the bioavailability of the drug by using suitable carrier systems based on the formation of supramolecular complexes (30). Thus, the widely spread application of CD in pharmaceutical formulations to enhance drug solubilization (31) further reinforces our interest in the present topic.
MATERIALS AND METHODS
Chemicals
FM (molecular weight 250.2) was purchased from Sigma Chemical Co. (St. Louis, MO) and used as received. Phosphate buffer 10-2 M, pH 7.4 was prepared from reagent grade products. pH of the solutions were measured with a glass electrode. All solvents used (from Carlo Erba, Milan) in the isolation of the photoproducts and in the photochemical experiments were high performance liquid chromatography (HPLC) and analytical grade, respectively.
Instrumentation
Steady state absorption and induced circular dichroism spectra (ICD) were recorded with a Beckman 650 DU spectrophotometer and a Jasco J-615 dichrograph, respectively. The IH-, 1917- and 13C-nuclear magnetic resonance (NMR) spectra were recorded on a Varian Inova spectrometer at 499.9, 470.3 and 125.7 MHz, respectively. The 114 and 13C experiments were obtained in CD30D and/or CDCl3 using tetramethylsilane as an internal reference; definitive assignments of individual IH and 13C resonances of the photoproducts were supported by 'H-'H-correlated spectroscopy and pulsed field gradient heteronuclear single-quantum correlation experiments, together with the analysis of '3C-19F coupling constants. Fast atom bombardment (FAB) and electron impact (EI) mass spectra were obtained on a Fisons ZAB 2SE spectrometer.
HPLC was performed on a Hewlett-Packard 1100 chromatograph equipped with on-line photodiode array detector. The quantitative analysis of FM and its photoproducts was achieved on a LiChroCart RP-18 column (5 ILm packing, 4 X 250 mm Hewlett-Packard) eluting with a linear gradient of CH3CN in 0.01 M phosphate buffer (pH 7) from 0 to 75% in 25 min, at a flow rate of 1 mL min-'. The retention times of FM and the photoproducts 1, 2 and 3 were 16.7, 13.7, 17.0 and 15.0 min, respectively.
Retention times in HPLC analysis for the compounds in the presence of 10-2 M '3-CD were the same as described above. Moreover, a quantitative evaluation of the injected material assured that no inclusion products were retained by the column (see Table 2).
Irradiation conditions
Irradiations of FM were performed using monochromatic irradiation obtained from a Series 200 He-Cd 325 run laser (Liconix, Santa Clara, CA). The incident photon flux on 3 mL quartz cuvettes was ca 5 X 1015 quanta s-1. The experimental procedures of irradiation and the light intensity measurements have been described previously (32). On a preparative scale, the irradiation of FM was performed in 200 mL quartz vessel using a Rayonet photochemical reactor equipped with a variable number of "black light" phosphor lamps with an emission in the 310-390 nm range with a maximum at 350 nm.
The quantum yields related to the FM photodegradation and to the formation of its photoproducts were calculated at 325 nm, below the 15% conversion of the starting compound, from the following relation:
(D = (d[XI/dt)v/FI (1) where d[X]/dt is the initial rate of disappearance of FM or the initial rate of formation of the single photoproducts, v is the volume of the irradiated sample, F = 1-10-A is the fraction of photons absorbed by FM at the excitation wavelength and I is the light intensity (mol of photons min-[).
Photodegradation of FM
FM solutions (200 mL, 10-1 M) in phosphate buffer in the absence or in the presence of 10-2 M '3-CD were irradiated in the Rayonet reactor under nitrogen bubbling and continuous stirring. The irradiated solutions (ca 15% FM transformation, determined by HPLC) were extracted with diethyl ether (3 X 20 mL); the combined extracts were dried over anhydrous sodium sulfate and evaporated in vacuo at 40*C. An analysis of the extracted mixture was performed by thin layer chromatography (TLC), on precoated silica gel 60 F254 plates (Merck) using dichloromethane n-propanol 99:1 (vol/vol) and dichloromethane:cyclohexane 80:20 (vol/vol) for the experiments carried out in the absence and in the presence of P3-CD, respectively. In the former case the TLC analysis showed a main photoproduct, 1 (Rf = 0.13), and the starting compound (Rf = 0.80). In the latter case the photoproduct 1 (Rf = 0.11) was accompanied by the presence of two other photoproducts, 2 (Rf = 0.28) and 3 (Rf = 0.38) (starting compound, Rf = 0.2). On a preparative scale, the photoproduct 1 was obtained, from the organic extracts of FM solutions (10-^ M, 5000 mL) irradiated in the absence of '3-CD, by preparative liquid chromatography on a silica gel column (40-63 pm; 1.0 X 18 cm2) using dichloromethane:n-propanol 99:1 (vol/vol) as eluent. The photoproduct 2 and 3 were recovered from the organic extracts of FM solutions (10-4 M, 2000 mL) irradiated in the presence of '3-CD, carrying out a chromatographic separation on a silica gel column (40-63 Rm; 1.0 X 20 cm2) eluting with dichloromethane: cyclohexane 60:40 (vol/vol) (3, Rf = 0.24; 2, Rf = 0.18; FM, Rf = 0.13; 1, Rf = 0.0).
The photolysis of FM in the organic solvent was performed by irradiating 10-4 M solutions of FM under nitrogen bubbling and continuous stirring. The quantitative analysis of the irradiated mixture was performed by means of HPLC under the same experimental conditions described above.
Characterization of the photoproducts
Photoproduct 1: 2-methyl-N-[4-hydroxy-3-(trifluoromethyl)phenyl] propanamide. IH-NMR (CD30D): 6 1.19 (d, J = 7.0 Hz, 6H, CH2), 2.59 (septet, J = 7.0 Hz, IH, CH[CH3]2), 6.88 (d, JH-5.li-6 = 8.7 Hz, 1H, H-5), 7.54 (dd, JH,H-5 = 8.7 Hz, JH-6.H-2 = 2.2 Hz, 1H, H-6), 7.72 (d, JH-2,H-6 = 2.2 Hz, IH, H-2); selected IH-NMR (CDCl3) data: 6 5.63 (br s, 1H, OH), 7.09 (br s, lH, CONH). 13C-NMR (CD30D): 8 19.8 (CH[CH3]2), 36.9 (CH[CH3]2), 117.8 (q, JCCF = 30.8 Hz, C-3), 117.9 (C-5), 120.2 (q, JCCCF = 5.2 Hz, C-2), 125.2 (q, JCF = 271.0 Hz, CF3), 126.7 (C-6), 131.6 (C-1), 153.6 (C-4), 178.5 (CONH). 19F-NMR (CDC13): 8 15.61 (s, CF3). FAB-mass spectrometry (MS) (+) (3-nitrobenzyl alcohol as matrix): m/z 248 [M + H]+.
Photoproduct 2: 2-methyl-N-[4-nitroso-3-(trifluoromethyl)phenyl] propanamide. 'H-NMR (CD30D): b 1.22 (d, J = 6.9 Hz, 6H, CH[CHj]2), 2.69 (septet, J = 6.9 Hz, 1H, CH[CH3]2), 6.41 (d, JH-5.H-6 = 9.0 Hz, 1H, H-5), 7.83 (dd, JHAH-5 = 9.0 Hz, JH-6,H-2 = 2.2 Hz, 1H, H-6), 8.46 (d, JH-2,H-6 = 2.2 Hz, 1H, H-2); selected IH-NMR (CDC13) data: 8 7.56 (br s, CONH). I-IC-NMR (CD30D): 6 19.6 (CH[CH3]), 37.3 (CH[CH3]), 109.3 (C-5), 117.3 (q, JCCcF = 6.0 Hz, C-2), 122.2 (C-6), 125.9 (q, JCF = 273.4 Hz, CF3), 134.9 (q, JccF = 32.5 Hz, C3), 147.5 (C-1), 158.9 (C-4), 179.2 (CONH). 19F-NMR (CDCI,): 6 21.10 (s, CF3). FAB-MS (+) (3-nitrobenzyl alcohol as matrix): m/z 261 [M + Hj+.
Photoproduct 3: 4-nitro-3-(trifluoromethyl)aniline. IH-NMR (CDC13): 8 4.46 (br s, 2H,NH2), 6.78 (dd, JH-6,H-5 = 8.8 Hz, JH-6,1-2 = 2.6 Hz, III, H-6), 6.98 (d, JH-2,11-6 = 2.6 Hz, lH, H-2), 7.97 (d, JH-5,14-6 = 8.8 Hz, 1H, H-5). 19F-NMR (CDC13): 6 16.34 (s, CF3). FAB-MS (+) (glycerol as matrix): m/z 207 [M + H]+. EI-MS (70 eV, 60C): rn z (rel. int. %) 206 [M]' (25.5), 176 [M - NO]" (28.9), 167 (18.9), 160 [M - NOz+ (21.1), 125 [176 - CHF2. (20.0), 69 [CF3]+ (100).
RESULTS AND DISCUSSION
Absorption spectra and electronic states of FM
The absorption spectrum of FM in phosphate buffer solution pH 7.4 (Fig. 1) is characterized by a broad band with maximum at 300 nm (E = 6200 M-' cm-1) and a weak tail extending beyond 400 nm. A significant shift toward shorter wavelengths was observed as the solvent polarity decreased. In particular, the main band shifted to 296 mn in methanol, 294 nm in isopropanol and 278 nm in cyclohexane. In order to asses the nature of the excited states of FM, we performed semiempirical quantum mechanical calculations by means of the Zerner intermediate neglect of differential overlap/spectroscopic method (Hyperchem software package), starting from a ground-state geometry optimized through the MM+ molecular mechanics routine (33). The results obtained are reported in Table 1.
The first sizeable singlet, responsible for the absorption band at 300 nm in aqueous solution, is calculated at 91.3 kcal mol-I as S3 (Tr,iT*). The blueshift observed in the solvent with decreasing polarity is in agreement with the fact that the main band has contributions from a ir,7r* state which is more polar than the ground state. As it can be noticed from Table 1, the lowest excited triplet state T, has a 7,,rr* nature and lies ca 10 kcal below the n,7r* triplet T, contrary to nitrobenzene, chosen as a comparison compound, where the lowest excited triplet has an n,,rr* character.
The presence of the CF3 substituent plays a key role in determining the geometry of the FM ground state as well as the energies of the excited states. As reported in Fig. 2 the FM structure optimized through MM+ calculations shows clearly that the nitro group is placed almost perpendicularly to the aromatic ring and, as a consequence, not conjugated with the latter. Both steric and electronic factors, due to the presence of the CF; substituent, can play dominant roles in determining such geometry. The lack of conjugation between the nitro group and the aromatic plane was further confirmed by the marked blueshift (ca 40 nm) of the absorption maximum of FM if compared to that of the analog compound without the CF3 group.
Photoreactivity in homogeneous media
Figure 3 shows the spectral changes observed in nitrogen saturated solution of FM 10-4 M in phosphate buffer pH 7.4 at different irradiation times upon 325 nm light excitation. As confirmed by the analysis of the irradiated mixture (see "Experimental") the spectral changes observed are consistent with the almost exclusive formation of 1 (see Scheme 1) as the main stable photoproduct. The photodegradation quantum yield of FM in these experimental conditions, calculated through HPLC analysis, was (-Fm = 3.5 X 10-3.
Even when the irradiation of FM was performed in anaerobic conditions in solvents with hydrogen-donating ability better than water such as methanol and isopropanol, 1 was always the main photoproduct. At a first glance, the lack in these solvents of clear evidence for any product originated by either an expected photoreduction of the nitro group or predictable cleavage of the amide bond might seem quite surprising. Indeed, although formed with low quantum efficiency, such photoproducts are commonly observed in the case of nitro aromatic (34-39) and aryl amide derivatives (38-42). This apparent incongruity can be understood on the basis of the chemical structure of FM. As discussed above, because of the presence in ortho of the CF3 substituent the nitro group is placed almost perpendicularly to the aromatic plane and, as a consequence, not conjugated with the latter. As pictorially represented in Scheme 1, such a twisted geometry makes the p orbital of the oxygen atom having a constructive overlap with the adjacent p orbital of the aromatic ring in the ground state. This geometry is believed to determine an n,7r* excited state characterized by a low biradical character and, as a consequence, by a very low photoreactivity towards hydrogen abstraction (H-abstraction) even in hydrogen-donating solvents.
In light of this view, the formation of 1 can be rationalized on the basis of the well-known nitro to nitrite rearrangement followed by rupture of the O-N bond to generate a phenoxy radical (Scheme 1). According to literature data (43-46) the out of plane geometry of the nitro group and the consequent orbital overlap are prerequisites to observe such a type of intramolecular photorearrangement. In this connection, it should be noted that although the nitrite isomer has never been identified it is widely regarded as a logical intermediate (45). Elegant studies performed by Hamanoue et al. (46) demonstrated that the nitro to nitrite photorearrangement occurs likely from an upper excited triplet state with an n,7r* character located at higher energy with respect to the lowest Tr,,IT* triplet state. This picture accords well with our results concerning both the nature and the energies of the low-lying triplet states of FM (see previous section).
FM-P-CD inclusion complex
The absorption spectrum of FM was slightly affected by the addition of [-CD (data not shown). A weak hypochromic effect accompanied by the lack of any clear isosbestic point was in fact observed upon addition of the macrocycle up to 10-2 M. Although this observation was indicative for some type of association, the spectral changes were too small to obtain any quantitative information on the host-guest system through this spectroscopic technique. A much more clear confirmation for the incorporation of FM in the [-CD cavity was provided by ICD. As shown in Fig. 4, the ICD signal arises as a consequence of the complexation of the nonchiral drug with the optically active host molecule. The ICD spectrum clearly shows a negative signal centered at -425 nm and a larger, positive signal at -350 nm. On the basis of the calculated energies, one can attribute the negative signal to the lowest n,iT* states, centered mainly on the N02 group, whereas the positive signal arises from the first allowed ir,7r* transition. Inspection of the sign of the ICD bands affords some hints on the structure of the complex formed even in absence of more refined calculations (47).$
In particular the effect of geometry of the N02 group (planar or perpendicular to the ring) on the sign of the ICD of the first band was investigated through a calculation of the m-l term (48,49), which appears relevant for an isolated n,Tr* transition centered on this group. It was found that the different symmetry of the magnetic moment in the two different situations plays a decisive role, giving rise to a fairly large negative rotatory power for the planar geometry and to a small but positive term for the perpendicular one. On the other hand, the ICD band of the ir,,rr* state, centered on the phenyl ring, is calculated positive through the f-ll electric dipole-dipole term. This argument support well the model invoked to explain the photoreactivity of FM in P-CD (see below).
The nonlinear analysis of the dependence of the ICD signals on the [-CD concentration (Fig. 4, insets) was performed according to (Eq. 2) (corresponding to conditions where the [-CD concentrations are in excess with respect to the guest concentration):
ICD = 100c0K,,,[R-CD]/(l + Kas[13-CD]) (2)
where co is the initial concentration of FM, is the molar ellipticity of the complex and Kris is the association constant. We have preferred to apply a nonlinear fit because in this way the data are weighted in a manner that reflects better its quality. The results of the plot are consistent with a 1:1 association constant, Kris = 300 50 M-1. This value compares well with those reported both for similar aryl amide (50-54) and nitro aromatic derivatives (55). In this regard, it should be noted that although the ICD data fit the equation that considers only a 1:1 complex, the lack of any isosbestic point observed in the absorption spectra upon addition of [CD indicate that more than one type of host-guest complex could be formed. Thus, the association constant obtained might be related to different complexes that cannot be distinguished by either absorption or ICD spectroscopy. This hypothesis is not unprecedented. Actually, the formation of different inclusion complexes with [-CD characterized by 1: 1 stoichiometry but different inclusion geometry is quite common for both aryl amide (51-54) and nitro aromatic derivatives (55).
Photoreactivity in P-CD environment
Figure 5 shows the spectral changes observed in a nitrogensaturated solution of FM in the presence of 10-2 M '3-CD upon 325 nm light excitation. A remarkably different spectral behavior is evident with respect to that observed in the absence of the macrocycle (Fig. 3). As confirmed by both quantitative and qualitative analyses of the irradiated mixture, the observed spectral variations are consistent with very significant changes in the nature and in the efficiency of the photodegradation pathways of FM. The formation of 1 was indeed accompanied by two new photoproducts, 2 and 3, resulted from photoreduction of the nitro group and photocleavage of the amide bond, respectively (Scheme 2). Furthermore, the photodegradation quantum yield of FM in the presence of the macrocycle increased by ca 20-fold with respect to that found in its absence. The overall results concerning the photoreactivity of FM in aqueous solution in the presence of O3-CD and, for the sake of comparison, in its absence are summarized in Table 2.
At first sight, we were surprised to observe the formation of the photoreduction product 2 in these experimental conditions. Indeed, the origin of such a compound cannot be roughly rationalized on the basis of either the presence of the easily abstractable hydrogen atoms of the macrocycle or the low polarity of the cavity when compared with water. As outlined earlier, the irradiation of FM performed in solvents such as either methanol or isopropanol, characterized by hydrogen-donating properties better than water and polarity not too much different than [-CD, gave indeed the almost exclusive formation of 1. Moreover, only this photoproduct was also observed when the irradiation of FM was performed in aqueous solution in the presence of 0.1 M glucose.
A possible hypothesis to explain the photogeneration of 2 could be consistent with "cage photoreactions" triggered by structural changes of FM upon incorporation in the O-CD cavity. A less perpendicular geometry of the nitro group with respect to the aromatic ring, caused by steric constrains and specific weak interactions (i.e. H-bond involving the CF3 and/or N02) with P-CD, would account well for the formation of the nitroso derivative 2. Such changes in the perpendicularity of the nitro group would lead in fact to a lessextended overlap of the p atomic orbital of oxygen with the adjacent orbital of the aromatic ring. The consequence of this type of conformation is reflected in a logical increase of the biradical character of the n,iT* triplet and a consequent higher ability of this latter in abstracting hydrogen in the presence of a suitable H-donor. Under these conditions, an intermolecular H-abstraction photoprocess involving the nitro group and the available hydrogen atoms of the '-CD cavity could become a significant photoprocess, thus giving rise to the formation of 2 according to the very well-known mechanistic pathways (34-39). Moreover, a reasonable decrease in the energy gap between the two low-lying n,rr* and iT,7r* triplet states due to the low polarity of the p-CD cavity might also play a key role for the increased photoreactivity. Finally, it is noteworthy that the quantum yield related to the formation of 2 (Table 2) accords well with the values commonly observed in the case of H-abstraction processes from the n,,rt* triplet of the nitroaromatic compounds in good hydrogen-donating solvents (34,36).
NMR and UV spectroscopic investigations do not provide unambiguous information to ascertain the structure of the inclusion complex. Nevertheless, the sequence of signs shown by the ICD spectrum, discussed in the previous section, leads to conclude that a more coplanar structure of FM in the P-CD appears highly probable.
As reported in Scheme 2, formation of photoproduct 3, in addition to 2 and 1, occurred when FM was irradiated in the presence of P-CD. Taking into account that the photocleavage of the amide bond is favored by a decrease of the solvent polarity (51,52,56), it would be conceivable to attribute the formation of 3 to the low polarity of the '3-CD cavity if compared to the aqueous solution. The absolute lack of 3 noticed in solvents with lower polarity than water, such as methanol and isopropanol (see "Photoreactivity in homogeneous media"), rules out this hypothesis. Further, a hydrolytic generation of 3 due to a potential catalytic effect of the P-CD in cleaving the amide bond (16,55) was also discarded. No degradation of FM at all was in fact observed after stirring a FM-[3-CD solution for 5 h at 40'C. On the basis of these results, the formation of 3 in the presence of P-CD might also reflect the conformational changes of the nitro group proposed earlier. Indeed, its tendency toward a less-perpendicular geometry in the [3-CD environment, would make this group much more conjugated with the aromatic ring, therefore leading to a weakening of the amide bond and favoring the cleavage of the latter. As ascertained well, a singlet excited state is the more likely responsible for these types of photoreaction (40,41,51-54). It is also noteworthy that no formation of isopropyl 2-amino-5-nitro4-(trifluoromethyl)phenyl ketone, the ortho isomer of FM expected by photo-Fries rearrangement after the amide bond photocleavage, was noticed. Since the formation of orthol para isomeric products is quite a common process for similar compounds (38,39,50-54) our results suggest that the photocleavage reaction occurs mainly in the cavity. Indeed, the acyl radical produced by the amide cleavage is big enough to be sterically hindered by the macrocycle in giving the ortho isomer of FM.
As shown in Table 2, the formation of 1 in the experiment performed in the presence of 3-CD occurs with a quantum yield higher than that observed in water solution. This fact clearly suggests that the molecules of FM not included in the cavity (ca 30%) cannot be the only ones responsible for the formation of 1, otherwise a lower efficiency in the formation of this photoproduct would have been expected. According to literature data regarding the formation of similar products in CD cages (51-54), the change of polarity may be responsible only in part for the increase of (DI. Then, we believe that an important part of 1 is formed from the recombination of the phenoxy radicals that are not in the solvent cage but trapped in the cavity of the macrocycle. As proposed by Veglia and de Rossi (52), under these conditions the competition with H-abstraction becomes more favorable than in aqueous solution given that in the interior of the [-CD cavity there are 14 available hydrogen atoms bonded to secondary carbons and close to the radical center.
This result stimulates a further comment. Since the formation of 1 also takes place in the P-CD environment, a constructive overlap between the p orbital of the oxygen with the adjacent p orbital of the aromatic ring could still be present even in the included compound. In this regard, the photoreduction pathway to form 2 and the nitro-nitrite photorearrangement to form 1 may reflect competitive photoprocesses, occurring from the same host-guest complex, independent photoprocesses, taking place from two different complexes with different inclusion geometry, or both. As reported in the case of similar aryl amide and nitro aromatic derivatives (53-55), the proposed scenario is quite common when more than one type of inclusion complex is formed. In these cases, the reactivity of complexed substrates is very much determined by the structure of the complexes whose orientation within the cavity is of great importance.
CONCLUSIONS
The photochemistry of the FM in homogeneous media provides an example of nitro to nitrite intramolecular photorearrangement triggered by the twisted conformation of the nitro group. Such an out of plane geometry is responsible for the low biradical character of the n,7r* triplet state of the drug reflected in the negligible photoreactivity towards Habstraction even in hydrogen-donating solvents.
FM incorporates into the [3-CD cavity. From a strict chemical point of view the overall photochemistry observed in the presence of the macrocycle represents an example of how the localization of a guest molecule in a restricted microenvironment can lead to dramatic changes in both the efficiency and the nature of the photochemical deactivation pathways. The photoreactivity of the guest molecule cannot be simply rationalized on the basis of the polarity of the host molecule and on its role as a reactant. The occurrence of a structural modification, as suggested by the ICD spectrum and by the nature of the photoproducts, appears more probable.
The present report can also be of biological relevance. Actually, given the potential of CD in providing an useful model to mimic the interactions of the drug with hydrophobic biological sites and by taking into account that all the photoproducts formed in P-CD are mediated by radical pathways, the obtained results represent a good step forward in understanding the recently reported phototoxic effects displayed by FM. Incorporation or localization of the drug in specific pockets of a biosubstrate with particular hydrophobicity and in the presence of steric constrains and specific interactions, could lead in fact to new and more efficient photodegradation pathways, with a significant increase in the photoproduction of radical species.
Finally, in light of the much lower photostability of the FM-P-CD inclusion complex if compared with the free molecule, the study presented herein suggests that the use of CD, or similar kinds of drug/carrier system, could not be the more appropriate approach to enhance the drug solubility. Acknowledgements-Partial financial supports from MURST in the framework of the "Programmi di Ricerca di Rilevante Interesse Nazionale" (project: Mechanisms of Photoinduced Processes in Homogeneous Media and in Complex Matrices) and from the Progetto "ProprietA Chimico-Fisiche dei medicamenti e loro sieurezze d'uso" of the ISS is gratefully acknowledged. We wish to thank Dr. F. Vargas for sharing with us a preprint of his manuscript.
1[Posted on the website on 22 November 2000.
,Abbreviations: O-CD, 0-cyclodextrin; El, electron impact; FAB, fast atom bombardment; FM, flutamide; H-abstraction, hydrogen abstraction; HPLC, high performance liquid chromatography; ICD, induced circular dichroism; MS, mass spectrometry; NMR, nuclear magnetic resonance; TLC, thin-layer chromatography.
$Unfortunately, we could not perform Monte Carlo theoretical calculation of the inclusion complex due to problems related to the parametrization of the N02 group.
REFERENCES
1. Schellhammer, P., R. Sharifi and N. Block (1995) A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology 45, 745-752.
2. Ornstein, D. K. (1998) Combined finasteride and flutamide therapy in men with advanced prostate cancer. Urology 48, 901905.
3. Mahler, C., J. Verhelst and L. Denism (1998) Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer. Clin. Pharmacokinet. 34, 405-417.
4. Leroy, D., A. Dompmartin and C. Szczurko (1996) Flutamide photosensitivity. Photodermatol. Photoimmunol. Photomed. 12, 216-218.
5. Zabala, R., J. Gardeazabal and D. Manzano (1995) Photosensibilidad por flutamida. Acta Dermosifiliogr. 86, 323-325.
6. Vilaplana, J., C. Romaguera, A. Azon and M. Lecha (1998) Flutamide photosensitivity-residual vitiliginous lesions. Contact Dermatitis 38, 68-70.
7. Reid, M. B. and L. M. Glode (1998) Flutamide induced lupus. J. Urol. 159, 2098-2101.
8. Yokote, R., Y. Tokura, N. Igarashi, 0. Ishikawa and Y. Miyachi (1998) Photosensitive drug eruption induced by flutamide. Eur. J. DermatoL 8, 427-429.
9. Borroni, G., V. Brazzelli, F. Baldini, F. Borghini, M. R. Gaviglio, B. Beltrami and G. Nolli (1998) Flutamide-induced pseudoporphyria. Br. J. Dermatol. 138, 711-712.
10. Vargas, F., C. Rivas, H. Mendez, A. Fuentes, G. Fraile and M. Velasquez Photochemistry and phototoxicity studies of flutamide, a phototoxic anti-cancer drug. J. Photochem. Photobiol. B: Biol. (In press)
11. Bortolus, P. and S. Monti (1996) Photochemistry in cyclodextrin cavities. Adv. Photochem. 21, 1-133.
12. Bender, M. L. and M. Komiyana (1978) Cyclodextrin Chemistry. Springer, Berlin.
13. Szejtli, J. and T. Osa (1996) Cyclodextrins Compren.sive Supramolecular Chemistry. Elsevier, New York.
14. Balzani, V. and L. De Cola (1992) Supramolecular Chemistry. Kluwer, Dordrecht.
15. Sztejtli, J. (1982) Cyclodextrins and their Inclusion Complexes. Akademiai Kiado, Budapest.
16. Saenger, W. (1980) Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Int. Ed. Engl. 19, 344-362.
17. Wenz, G. (1994) Cyclodextrins as building blocks for supramolecular structures and functional units. Angew. Chem. Int. Ed. Engl. 33, 803-822.
18. Uekama, K., F. Hirayama and T. Ire (1998) Cyclodextrin drug carrier systems. Chem Rev. 98, 2045-2076.
19. Ramamurthy, V., R. G. Weiss and G. S. Hammond (1993) A model for the influence of organized media on photochemical reactions. Adv. Photochem. 18, 67-234.
20. Ramamurthy, V. (1991) Photochemistry in Organized and Constrained Media. VCH Publishers, New York.
21. Kalyanasundaram, K. (1987) Photochemistry in Microheterogeneous Systems. Academic Press. Orlando.
22. Monti, S., S. Sortino, G. De Guidi and G. Marconi (1998) Supramolecular photochemistry of 2-(3-benzoylphenyl)propionic acid (Ketoprofen). A study in the -cyclodextrin cavity. New J. Chem. 22, 599-604.
23. Sortino, S., G. De Guidi, G. Marconi and S. Monti (1998) Triplet photochemistry of suprofen in aqueous environment and in the P-cyclodextrin inclusion complex. Photochem. Photobiol. 67, 603-611.
24. Sortino, S., J. C. Scaiano, G. De Guidi and S. Monti (1999) Effect of [-cyclodextrin complexation on the photochemical and photosensitizing properties of tolmetin: a steady state and time resolved study. Photochem. Photobiol. 70, 549-556.
25. Jimenez, M. C., M. A. Miranda and R. Tormos (1995) Photodecarboxylation of 2-phenylpropionic acid in solution and induced within 0-cyclodextrin. Tetrahedron 51, 2953-2958.
26. Monti, S., S. Sortino, S. Encinas, G. Marconi, G. De Guidi and M. A. Miranda (1998) Photoprocesses in photosensitizing drugs containing a benzophenone-like chromophore. In: Drugs Photochemistry and Photostability (Edited by A. Albini and E. Fasani), pp. 150-161. The Royal Society of Chemistry, Cambridge.
27. De Guidi, G., G. Condorelli, L. L. Costanzo, S. Giuffrida, S. Monti, and S. Sortino (1998) Molecular mechanisms of photosensitization induced by drugs on biological systems and design of photoprotective systems. In: Drugs Photochemistry and Photostability (Edited by A. Albini and E. Fasani), pp. 194-210. The Royal Society of Chemistry Cambridge.
28. De Guidi, G., G. Condorelli, S. Giuffrida, G. Puglisi and G. Giammona (1993) Effect of -cyclodextrin complexation on the photohemolytic activity induced by ketoprofen and naproxen sensitization. J. Incl. Phenom. Mol. Rec. Chem. 15, 43-58.
29. Hoshino, T. H., K. Ishida, T. Irie, F. Hirayama and M. Yamasaki (1988) Reduction of photohemolytic activity of benoxaprofen by -cyclodextrin complexation. J. Incl. Phenom. 6, 415-423.
30. Adel, M. A., A. S. Geneidi, R. Ali Shoukri and I. Saad (1997) In vitro evaluation of flutamide-carrier systems. Pharmazie 52, 373-375.
31. Uekama, K., F. Hirayama and T. Irie (1998) Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045-2076.
32. De Guidi, G., R. Chillemi, L. L. Costanzo, S. Giuffrida, S. Sortino and G. Condorelli (1994) Molecular mechanism of drug photosensitization. Part 5. Photohemolysis sensitized by suprofen. J. Photochem. Photobiol. B: Biol. 23, 125-133.
33. Hyperchem package, Hypercube Inc., Waterloo Canada, 1996. 34. Hurley, R. and A. C. Testa (1966) Photochemical n-w* excitation of nitrobenzene. J. Am. Chem. Soc. 88, 4330-4332.
35. Barltropp, J. A. and N. J. Bunce (1968) Organic photochemistry. Part VIII. The photochemical reduction of nitrocompounds. J. Chem. Soc. 1467-1474.
36. Levy, N. and M. D. Cohen (1979) Photoreduction of 4-cyano1-nitrobenzene in propan-2-ol. J. Chem. Soc. Perkin Trans. II 553-558.
37. Bosca, F., M. A. Miranda, G. Serrano and F. Vargas (1998) Photochemistry and photobiological properties of dicloran, a prostharvest fungicide with photosensitizing side effects. Photochem. Photobiol. 67, 532-537.
38. Gilbert, A. and J. Baggot (1991) Essentials of Molecular Photochemistry. Blackwell Scientific, London.
39. N. J. Turro (1978) Modern Molecular Photochemistry. Benjamin/Cummings Publishing, Menlo Park.
40. Sandner, M. R. and D. J. Trecker (1967) On the mechanism of the photo-Fries reaction. J. Ant Chem. Soc. 89, 5725-5726.
41. Kalmus, C. E. and D. M. Hercules (1974) A mechanistic study of the photo-Fries rearrangement of phenyl acetate. J. Am. Chem. Soc. 96, 449-455.
42. Kobsa, H. (1962) Rearrangement of aromatic esters by ultraviolet radiation. J. Org Chem. 27, 2293-2298.
43. Chapman, 0. L., D. C. Heckert, J. W. Reasoner and S. P. Thackaberry (1960) Photochemical studies on 9-nitroanthracene. J. Am. Chem. Soc. 88, 5550-5554.
44. loki, Y. (1976) Aryloxyl radicals by photorearrangement of nitro-compounds. J. Chem. Soc. Perkin Trans. 11 1240-1242.
45. Hamanoue, K., M. Amano, M. Kimoto, Y. Kajiwara, T. Nakayama and H. Teranishi (1984) Photochemical reactions of nitroanthracene derivatives in fluid solutions. J. Am. Chem. Soc. 106, 5993-5997.
46. Dopp, D. (1995) Photochemical reactivity of the nitro group. In: Handbook of Organic Photochemistry and Photobiology. (Edited by W. M. Horspool and P. S. Song), pp. 1019-1062. CRC Press, Boca Raton.
47. Marconi, G. and B. Mayer (1997) Conformational and circular dichroism studies on cyclodextrin inclusion complexes. Pure Appl. Chem. 69, 779-783.
48. Schellman, J. A. (1968) Symmetry rules for optical rotation. Acc. Chem. Res. 1, 144-151.
49. Marconi, G. and J. Houben (1985) Magnetic circular dichroism spectra of azobenzene and its 4-amino and 4-nitro derivatives. J. Chem. Soc. Faraday Trans. 11 81, 975-983.
50. Symala, M. S., B. Nageswer Rao and V. Ramamurthy (1988) Modification of photochemical reactivity by cyclodextrin complexation: products selectivity in photo-Fries rearrangement. Tetrahedron 44, 7234-7242.
51. Veglia, A. V., A. M. Sanchez and R. H. de Rossi (1990) Changes of selectivity in the photo-Fries rearrangement of phenyl acetate induced by P-cyclodextrin. J. Org. Chem. 55, 4083-4086.
52. Veglia, A. V. and R. H. de Rossi (1993) [3-Cyclodextrin effects on the photo-Fries rearrangement of aromatic alkyl esters. J. Org. Chem. 58, 4941-4944.
53. Nassetta, M., R. H. de Rossi and J. J. Cosa (1998) Influence of cyclodextrins on the photo-Fries rearrangement of acetanilide. Can. J. Chem. 66, 2794-2798.
54. Chenevert, R. and R. Plante (1983) Photochemical rearrangement of acetanilide, benzanilide and ethyl phenyl carbonate in the presence of [-cyclodextrin. Can. J. Chem. 61, 1092-1095.
55. Tee, 0. S., C. Mazza and X. Du (1990) Chain length effects in the cleavage of aryl esters by cyclodextrins. Different transition states for m- and p-nitrophenyl alkanoates. J. Org. Chem. 55, 3603-3609.
56. Shizuka, H. and I. Tanaka (1968) Photochemistry of acetanilide. Quantum yields of the rearrangement and benzene photosensitized reaction. Bull. Chem. Jpn 41, 2343-2349.
S. Sortino*', S. Gluffridal, G. De Guldil, R. Chillemil, S. Petralial, G. Marconi% G. Condorellil and S. Sciuto,
'Dipartimento di Scienze Chimiche, Universit& di Catania, Catania, Italy and 21stituto di Fotochimica e Radiazioni d'Alta Energia, Bologna, Italy
Received 26 June 2000; accepted 11 October 2000
*To whom correspondence should be addressed at: Dipartimento di Scienze Chimiche, Universita di Catania, Viale Andrea Doria, 8 1-95125 Catania, Italy. E-mail: ssortino@dipchi.unict.it
Copyright American Society of Photobiology Jan 2001
Provided by ProQuest Information and Learning Company. All rights Reserved