Introduction
Folic acid, also known generically as folate or folacin, is a member of the B-complex family of vitamins, and works in concert with vitamin B 12. Folic acid functions primarily as a methyl-group donor involved in many important body processes, including DNA synthesis. Therapeutically, folic acid is instrumental in reducing homocysteine levels and the occurrence of neural tube defects. It may play a key role in preventing cervical dysplasia and protecting against neoplasia in ulcerative colitis. Folic acid also shows promise as part of a nutritional protocol to treat vitiligo, and may reduce inflammation of the gingiva. Furthermore, certain neurological, cognitive, and psychiatric presentations may be secondary to folate deficiency. Such presentations include peripheral neuropathy, myelopathy, restless legs syndrome, insomnia, dementia, forgetfulness, irritability, endogenous depression, organic psychosis, and schizophrenia-like syndromes.
Biochemistry
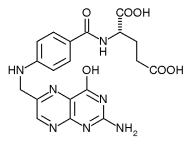
Folic acid is a water-soluble member of the B-complex family of vitamins. Folic acid is composed of three primary structures, a hetero-bicyclic pteridine ring, para-aminobenzoic acid (PABA), and glutamic acid. Because humans cannot synthesize this compound, it is a dietary requirement.
Although folic acid is the primary form of folate used in dietary supplements or fortified foods, it comprises only 10 percent or less of folates in the diet. Dietary folic acid, or the form naturally found in foods, is actually a complex and variable mixture of folate compounds, such as polyglutamate (multiple glutamate molecules attached) conjugate compounds, reduced folates, and tetrahydrofolates. Although folates are abundant in the diet, cooking or processing destroys these compounds. The best folate sources in foods are green, leafy vegetables; sprouts, fruits, brewer's yeast, liver, and kidney also contain high amounts of folates.
Pharmacokinetics
Human pharmacokinetic studies indicate folic acid has very high bioavailability, with large oral doses of folic acid substantially raising plasma levels in healthy subjects in a time- and dose-dependent manner. Subsequent to high-dose oral administration of folic acid (ranging from 25-1,000 mg/day), red blood cell (RBC) folate levels remain elevated for periods in excess of 40 days following discontinuation of the supplement. Folic acid is poorly transported to the brain and rapidly cleared from the central nervous system. The primary methods of elimination of absorbed folic acid are fecal (through bile) and urinary. (1-4)
After ingestion, the process of conversion of folic acid to the metabolically active coenzyme forms is relatively complex. Synthesis of the active forms of folic acid requires several enzymes, adequate liver and intestinal function, and adequate supplies of riboflavin (B2), niacin (B3), pyridoxine (B6), zinc, vitamin C, and serine. After the formation of the coenzyme forms of the vitamin in the liver, these metabolically active compounds are secreted into the small intestine with bile (the folate enterohepatic cycle), where they are reabsorbed and distributed to tissues throughout the body. Despite the biochemical complexity of this process, evidence suggests oral supplementation with folic acid is able to increase the body's pool of the active reduced folate metabolites (such as methyltetrahydrofolate) in healthy individuals. (5)
Enzyme defects, malabsorption or digestive system pathology, and liver disease can result in impaired ability to activate folic acid to the required coenzyme forms in the body. Evidence indicates some individuals have a severe congenital deficiency of the enzyme methyltetrahydrofolate reductase, which is needed to convert folic acid to the 5-methyltetrahydrofolate coenzyme form of the vitamin. The existence of milder forms of this enzyme defect is strongly suspected and likely interacts with dietary folate status to determine risk for some disease conditions. (6-10) In individuals with a genetic defect of this enzyme (whether mild or severe), greater dietary exposure to foods rich in folates and supplemental folates in the form of folinic acid or 5-methyltetrahydrofolate might be preferable to folic acid supplementation.
Mechanisms of Action
Folic acid's primary mechanisms of action are through its role as a methyl donor in a range of metabolic and nervous system biochemical processes, as well as being necessary for DNA synthesis. Serine reacts with tetrahydrofolate, forming 5,10-methylenetetrahydrofolate, the folate derivative involved in DNA synthesis. A methyl group is donated to cobalamin (B12) by 5-methyltetrahydrofolate, forming methylcobalamin. With the help of the enzyme methionine synthase, methylcobalamin donates a methyl group to the amino acid metabolite homocysteine, converting it to the amino acid methionine.
Methionine subsequently is converted to S-adenosylmethionine (SAMe), a methyl donor involved in numerous biochemical processes.
Deficiency States and Symptoms
Folic acid deficiency is considered to be one of the most common nutritional deficiencies. The following may contribute to a deficiency of folic acid: deficient food supply; defects in utilization, as in alcoholics or individuals with liver disease; malabsorption; increased needs in pregnant women, nursing mothers, and cancer patients; metabolic interference by drugs; folate losses in hemodialysis; and deficiencies in enzymes or cofactors needed for the generation of active folic acid. (11) Absorption of folic acid appears to be significantly impaired in HIV disease, irrespective of the stage of the disease. (12)
Signs and symptoms of folate deficiency include macrocytic anemia, fatigue, irritability, peripheral neuropathy, tendon hyper-reflexivity, restless legs syndrome, diarrhea, weight loss, insomnia, depression, dementia, cognitive disturbances, and psychiatric disorders. (13-18) Elevated plasma homocysteine can also indicate a dietary or functional deficiency of folic acid.
Clinical Indications
Anemia
Folic acid has a long history of use in conjunction with vitamin B 12 for the treatment of macrocytic anemia. Depending on the clinical status of the patient, the dose of folic acid required to reverse macrocytic anemia varies, but the therapeutic dose is usually 1 mg daily. Duration of therapy to reverse macrocytic anemia can be as short as 15 days after initiation of supplementation, or it may require prolonged supplementation.
Cervical Dysplasia
Research points to an association between folate status in adults and cervical dysplasia; (19-21) however, its role as an efficacious therapeutic intervention is unclear. One report suggests folic acid supplementation (10 mg folic acid for three months) reverses cervical dysplasia in women taking oral contraceptives. (22) In another study, 154 individuals with grade 1 or 2 cervical intraepithelial neoplasia were randomly assigned either 10 mg folic acid or placebo daily for six months. No significant differences were observed between supplemented and unsupplemented subjects regarding dysplasia status, biopsy results, or prevalence of human papilloma virus type-16 infection. (23) It is possible certain subsets of women (perhaps those with an oral contraceptive-induced deficiency) might be more amenable to treatment; however, additional research is required to clarify the therapeutic role of folic acid in cervical dysplasia.
Gout
There is no evidence demonstrating efficacy of folic acid supplementation in gout. Although some in vitro evidence suggests folate compounds are potent inhibitors of xanthine oxidase activity, (24) it appears pterin aldehyde, a photolytic breakdown product of folic acid, and not folic acid itself, is responsible for the observed inactivation of xanthine oxidase. (25)
Available evidence has shown no ability of supplemental folic acid in oral daily doses up to 1,000 mg to significantly lower serum urate concentration, or to decrease urinary urate or total oxypurine excretion in hyperuricemic subjects. (26)
Homocysteinemia
An abnormally high plasma level of homocysteine, the de-methylated derivative of the amino acid methionine, is an independent risk factor for cardiovascular disease. Elevated plasma homocysteine has been connected to increased risk of neural tube defects and other birth defects, as well as to schizophrenia, Alzheimer's disease, cognitive decline, osteoporosis, rheumatoid arthritis, kidney failure, and cancer. (27-31)
The activated coenzyme form of folic acid (5-methyltetrahydrofolate) is needed for optimal homocysteine metabolism, since it acts as a methyl donor, providing a methyl group to vitamin B12. The methylated form of vitamin B12 (methylcobalamin) subsequently transfers this methyl group to homocysteine. The result is a recycling of homocysteine to methionine, resulting in reduction in elevated plasma homocysteine.
In healthy subjects even low doses of folic acid can lower homocysteine levels. A dose of 250 mcg daily for four weeks reduced homocysteine an average of 11.4 percent in healthy 18- to 40-year-old women. A dose of 500 mcg daily for the same duration reduced levels an average of 22 percent. (32) In a separate study, 650 mcg daily for six weeks resulted in an average plasma homocysteine reduction of 41.7 percent. (33)
In subjects with cardiovascular disease, 800 mcg folic acid daily resulted in an average decrease in homocysteine levels of 23 percent, (27) while 2.5 mg daily resulted in an average decrease of 27 percent. (34) In subjects receiving the higher dose, 94 percent experienced some degree of reduction in homocysteine. (28) Evidence suggests individuals with higher initial homocysteine levels are likely to experience a greater reduction following folic acid supplementation. (34)
In addition to helping reduce blood levels of homocysteine, folic acid may also aid peripheral blood flow by increasing nitric oxide (NO) in vascular endothelial cells. Impaired endothelial NO activity is an early marker for cardiovascular disease, particularly atherosclerosis. In fact, most of the risk factors for atherosclerosis are associated with poor vasodilation due to insufficient NO production. Chronic, unopposed exposure of the vascular endothelium to homocysteine compromises the production of adequate amounts of NO, which leads to injury of the endothelial lining and the initiation/exacerbation of atherosclerosis and/or thrombus formation. Folic acid appears to improve NO synthesis by reducing plasma homocysteine levels, enhancing the availability of key endothelial NO cofactors, and reducing the production of superoxide anions, the net effect of which is improvement of peripheral blood flow. (35,36)
In a recent doubled-blind, placebo-controlled, crossover study of individuals with coronary heart disease, researchers found supplementation with high-dose folic acid (30 mg per day) improved blood flow to the heart muscle via the coronary arteries. Using positron emission tomography (PET scanning), researchers at Massachusetts General Hospital noted significant improvement in coronary blood flow with folic acid supplementation compared to placebo. The improvement was especially enhanced in areas of the heart that had shown reduced blood flow prior to supplementation. Folic acid supplementation also significantly lowered the study participants' blood pressure. The findings from this high-dose folate study demonstrate another significant way this nutrient benefits the cardiovascular system. (37)
Although excellent results have been achieved with folic acid monotherapy, available evidence suggests an additive effect exists between folic acid and vitamins B6, B12, and betaine with respect to lowering homocysteine levels. Combinations of these nutrients typically produce greater reductions in homocysteine than does folic acid alone. (27-29,38) Furthermore, the addition of vitamin C, L-arginine, tetrahydrobiopterin (B[H.sub.4]), and polyunsaturated fatty acids (PUFAs) has been suggested as a means of enhancing the effect of folic acid on endothelial NO production. (35)
Inflammatory Bowel Disease
Patients with inflammatory bowel disease (IBD) often have folate deficiencies, caused in part by the drug sulfasalazine, prescribed for IBD but also known to inhibit folate absorption. (39) Evidence suggests folic acid supplementation might lower the risk, in a dose-dependent fashion, of colonic neoplasia in patients with ulcerative colitis. A review of 99 ulcerative colitis (UC) patient records found folic acid supplementation was associated with a 62-percent decreased risk of neoplasia compared to patients not taking folate supplements. (39) In another similar study, the files of 98 UC patients disclosed dose-dependent protection from neoplasia by folic acid. The relative risk of developing neoplasia was 0.76 for 400 mcg folate and 0.54 for those taking 1 mg folate for at least six months compared to those not supplemented. (40)
Neuropsyehiatric Applications
Neuropsychiatric diseases encompass a number of neurological, cognitive, and psychiatric presentations that may be secondary to folate deficiency. Such presentations include dementia, schizophrenialike syndromes, insomnia, irritability, forgetfulness, endogenous depression, organic psychosis, peripheral neuropathy, myelopathy, and restless legs syndrome. (14-18)
Lower serum and RBC folate concentrations have an association with depression, and deficiency might predict a poorer response to some antidepressant medications. (30,41-47) Several studies have documented improvement in depression in some patients subsequent to oral supplementation with the coenzyme form of folic acid (methyltetrahydrofolate) at doses of 15-50 mg daily. (48,49) Folic acid (500 mcg per day) significantly improved the antidepressant action of fluoxetine in subjects with major depression. (50)
Limited evidence implies supplemental folic acid might positively affect morbidity of some bipolar patients placed on lithium therapy. (51)
A syndrome characterized by mild depression, permanent muscular and intellectual fatigue, mild symptoms of restless legs, depressed ankle jerk reflexes, diminution of vibration sensation in the legs, stocking-type hypoesthesia, and long-lasting constipation appears to respond to folic acid supplementation (5-10 mg per day for 6-12 months). (52)
Periodontal Disease
Folic acid can increase the resistance of the gingiva to local irritants and lead to a reduction in inflammation. A mouthwash containing 5 mg folate per 5 mL of mouthwash used twice daily for four weeks, with a rinsing time of one minute, appears to be the most effective manner of application. The effect of folate on gingival health appears to be moderated largely, if not totally, through a local influence. (53-44)
Pregnancy
Low dietary intake of folic acid increases the risk for delivery of a child with a neural tube defect (NTD). Periconceptional folic acid supplementation significantly reduces the occurrence of NTD. (56-62)
Supplemental folic acid intake during pregnancy results in increased infant birth weight and improved Apgar scores, along with a concomitant decreased incidence of fetal growth retardation and maternal infections. (63-65)
Vitiligo
In some individuals, administration of folic acid appears to be a rational aspect of a nutritional protocol to treat vitiligo. Degrees of re-pigmentation ranging from complete re-pigmentation in six subjects and 80-percent re-pigmentation in two subjects were reported in eight individuals who followed a three-year protocol with a dosage of 2 mg folic acid twice daily, 500 mg vitamin C twice daily, and intramuscular injections of vitamin B 12 every two weeks. (66)
A two-year study using a combination of folic acid, vitamin B12, and sun exposure for treatment of vitiligo reported positive results. One hundred patients with vitiligo were treated, with re-pigmentation occurring in 52 subjects. Total re-pigmentation was seen in six patients and the spread of vitiligo was halted in 64 percent of the patients. Re-pigmentation was most evident on sun-exposed areas. (67)
Drug-Nutrient Interactions
A number of drugs can interfere with the pharmacokinetics of folic acid.
Cimetidine and antacids appear to reduce folate absorption. (68)
Sulfasalazine interferes with folic acid absorption and conversion to the active form. (69) Supplementation with folic acid (15 mg/day for one month) prevents folate deficiency in patients with inflammatory bowel disease treated with sulfasalazine. (70)
Continuous long-term use of acetaminophen and aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs appears to increase the body's need for folic acid. (69)
Although the mechanism is unclear, anticonvulsants, antituberculosis drugs, alcohol, and oral contraceptives produce low serum and tissue concentrations of folate. (69,71)
Folic acid reduces elevated liver enzymes induced by methotrexate therapy in rheumatoid arthritis; however, it had no effect on the incidence, severity, and duration of other adverse events. (72)
Folic acid supplementation prevents nitric oxide synthase dysfunction induced by continuous nitroglycerin use. (73)
Anti-seizure medications, including carbamazepine and phenobarbital, appear to utilize folic acid during hepatic metabolism. Folic acid supplementation can increase metabolism of these drugs, thus lowering blood levels of the drugs and possibly resulting in breakthrough seizures. Initiating folic acid therapy after starting these drugs in individuals should be done with caution. (74)
The anticonvulsant drugs phenytoin and valproic acid appear to interfere with folate absorption. (75) Folic acid supplementation, at a time of day other than when taking an anticonvulsant, may be helpful to prevent deficiency.
There is conflicting information regarding the effects of folate supplementation in individuals treated with antifolate medications such as methotrexate (MTX) and 5-fluorouracil (5-FU). There is evidence folic acid might inhibit the activity of these drugs, although in some cases it may increase activity. In fact, the folic acid metabolite, folinic acid (also known as 5-formyltetrahydrofolate and leucovorin), is often used to "rescue" normal tissue after MTX or 5-FU therapy. Folic acid supplementation does not appear to interfere with methotrexate's anti-arthritic or anti-inflammatory activity. Since these medications are used to treat a wide range of malignant and nonmalignant disorders, indiscriminate use of folates should be avoided until further investigation is conducted.
Nutrient-Nutrient Interactions
Some concern exists that supplementation with high doses of folic acid could mask a vitamin B12 deficiency, resulting in neurological injury secondary to undiagnosed pernicious anemia. If there is any possibility of B12-induced anemia in an individual needing folate therapy, dual therapy with B12 and folate should be administered.
Some authors have suggested folic acid supplements might interfere with intestinal zinc absorption: however, doses as high as 15 mg folic acid daily do not appear to have any significant effect on zinc status in healthy, non-pregnant subjects. (74)
Side Effects and Toxicity
In doses typically administered for therapeutic purposes, folic acid is considered non-toxic. At doses of 15 mg daily and above, gastrointestinal complaints, insomnia, irritability, and fatigue have been mentioned as occasional side effects.
Folic acid is considered safe during pregnancy, with an established recommended intake of 800 mcg daily.
Dosage
The dose of folic acid required varies depending on the clinical condition. For lowering homocysteine, a minimum dose of 800 meg daily is generally used. The most common therapeutic dose is in the range of 1-3 mg daily. Doses greater than 10 mg daily have been used in conditions such as cervical dysplasia.
Dosages of over-the-counter folic acid supplements are restricted to no more than 800 meg of folic acid per serving, although prescription forms of folic acid are available in higher doses.
References
(1.) Zettner A, Boss GR, Seegmiller JE. A long-term study of the absorption of large oral doses of folic acid. Ann Clin Lab Sci 1981 ; 11:516-524.
(2.) Schuster O, Weimann H J, Muller J, et al. Pharmacokinetics and relative bioavailability of iron and folic acid in healthy volunteers. Arzneimittelforschung 1993;43:761-766. [Article in German]
(3.) Gregory JF 3d, Bhandari SD, Bailey LB, et al. Relative bioavailability of deuterium-labeled monoglutamyl tetrahydrofolates and folic acid in human subjects. Am J Clin Nutr 1992;55:1147-1153.
(4.) Levitt M, Nixon PF, Pincus JH, Bertino JR. Transport characteristics of folates in cerebrospinal fluid; a study utilizing doubly labeled 5-methyltetrahydrofolate and 5-formyltetrahydrofolate. J Clin Invest 1971;50:1301-1308.
(5.) Priest DG, Schmitz JC, Bunni MA. Accumulation of plasma reduced folates after folic acid administration. Semin Oncol 1999;26:$38-$41.
(6.) Yates JR, Ferguson-Smith MA, Shenkin A, et al. Is disordered folate metabolism the basis for the genetic predisposition to neural tube defects? Clin Genet 1987;31:279-287.
(7.) Lussier-Cacan S, Xhignesse M, Piolot A, et al. Plasma total homocysteine in healthy subjects: sex-specific relation with biological traits. Am J Clin Nutr 1996;64:587-593.
(8.) Kluijtmans LA, Van den Heuvel LP, Boers GH, et al. Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet 1996;58:35-41.
(9.) Whitehead AS, Gallagher P, Mills JL, et al. A genetic defect in 5,10 methylenetetrahydrofolate reductase in neural tube defects. QJM 1995;88:763-766.
(10.) Ulvik A, Evensen ET, Lien EA, et al. Smoking, folate and methylenetetrahydrofolate reductase status as interactive determinants of adenomatous and hyperplastic polyps of colorectum. Am J Med Genet 2001;101:246-254.
(11.) Halsted CH. The intestinal absorption of dietary folates in health and disease. J Am Coll Nutr 1989;8:650-658.
(12.) Revell P, O'Doherty MJ, Tang A, Savidge GF. Folic acid absorption in patients infected with the human immunodeficiency virus. J Intern Med 1991;230:227-231.
(13.) Botez MI. Folate deficiency and neurological disorders in adults. Med Hypotheses 1976;2:135-140.
(14.) Audebert M, Gendre JP, Le Quintrec Y. Folate and the nervous system (author's transl). Sem Hop 1979;55:1383-1387. [Article in French]
(15.) Young SN, Ghadirian AM. Folic acid and psychopathology. Prog Neuropsychopharmacol Biol Psychiatry 1989; 13:841-863.
(16.) Metz J, Bell AH, Flicker L, et al. The significance of subnormal serum vitamin B12 concentration in older people: a case control study. J Am Geriatr Soc 1996;44:1355-1361.
(17.) Quinn K, Basu TK. Folate and vitamin B 12 status of the elderly. Eur J Clin Nutr 1996;50:340-342.
(18.) Fine EJ, Soria ED. Myths about vitamin B12 deficiency. South Med J 1991;84:1475-1481.
(19.) Liu T, Soong S J, Wilson NP, et al. A case control study of nutritional factors and cervical dysplasia. Cancer Epidemiol Biomarkers Prev 1993;2:525-530.
(20.) Grio R, Piacentino R, Marchino GL, Navone R. Antineoblastic activity of antioxidant vitamins: the role of folic acid in the prevention of cervical dysplasia. Panminerva Med 1993;35:193-196.
(21.) Kwasniewska A, Tukendorf A, Semczuk M. Folate deficiency and cervical intraepithelial neoplasia. Eur J Gynaecol Oncol 1997;18:526-530.
(22.) Butterworth CE Jr, Hatch KD, Gore H, et al. Improvement in cervical dysplasia associated with folic acid therapy in users of oral contraceptives. Am J Clin Nutr 1982;35:73-82.
(23.) Zarcone R, Bellini P, Carfora E, et al. Folic acid and cervix dysplasia. Minerva Ginecol 1996;48:397-400. [Article in Italian]
(24.) Lewis AS, Murphy L, McCalla C, et al. Inhibition of mammalian xanthine oxidase by folate compounds and amethopterin. J Bio Chem 1984;259:12-15.
(25.) Spector T, Ferone R. Folic acid does not inactivate xanthine oxidase. J Biol Chem 1984;259:10784-10786.
(26.) Boss GR, Ragsdale RA, Zettner A, Seegmiller JE. Failure of folic acid (pteroylglutamic acid) to affect hyperuricemia. J Lab Clin Med 1980;96:783-789.
(27.) Landgren F, Israelsson B, Lindgren A, et al. Plasma homocysteine in acute myocardial infarction: homocysteine-lowering effect of folic acid. J Intern Med 1995;237:381-388.
(28.) Wilcken DE, Dudman NP, Tyrrell PA. Homocystinuria due to cystathionine beta-synthase deficiency--the effects of betaine treatment in pyridoxine-responsive patients. Metabolism 1985;34:1115-1121.
(29.) Dudman NP, Wilcken DE, Wang J, et al. Disordered methionine/homocysteine metabolism in premature vascular disease. Its occurence, cofactor therapy, and enzymology. Arterioscler Thromb 1993;13:1253-1260.
(30.) Fava M, Borus JS, Alpert JE, et al. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 1997; 154:426-428.
(31.) Miller AL, Kelly GS. Homocysteine metabolism: nutritional modulation and impact on health and disease. Altern Med Rev 1997;2:234-254.
(32.) Brouwer IA, van Dusseldorp M, Thomas CMG, et al. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomised trial. Indian Heart J 2000;52:S53-S58.
(33.) Ubbink JB, Vermaak WJ, van der Merwe A, et al. Vitamin requirements for the treatment of hyperhomocysteinemia in humans. J Nutr 1994;124:1927-1933.
(34.) Wald DS, Bishop L, Wald NJ, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med 2001;161:695-700.
(35.) Das UN. Folic acid says NO to vascular diseases. Nutrition 2003;19:686-692.
(36.) Hyndman ME, Verma S, Rosenfeld RJ, et al. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am J Physiol Heart Circ Physiol 2002;282:H2167-H2172.
(37.) Tawakol A, Migrino RQ, Aziz KS, et al. High-dose folic acid acutely improves coronary vasodilator function in patients with coronary artery disease. J Am Coll Cardiol 2005;45:1580-1584.
(38.) Wilcken DE, Wilcken B, Dudman NP, Tyrrell PA. Homocystinuria--the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med 1983;309:448-453.
(39.) Lashner BA, Heidenreich PA, Su GL, et al. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology 1989;97:255-259.
(40.) Lashner BA, Provencher KS, Seidner DL, et al. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology 1997;112:29-32.
(41.) Abou-Saleh MT, Coppen A. Serum and red blood cell folate in depression. Acta Psychiatr Scand 1989;80:78-82.
(42.) Alpert JE, Fava M. Nutrition and depression: the role of folate. Nutr Rev 1997;55:145-149.
(43.) Wesson VA, Levitt AJ, Joffe RT. Change in folate status with antidepressant treatment. Psychiatry. Res 1994;53:313-322.
(44.) Papakostas GI, Petersen T, Mischoulon D, et al. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry 2004;65:1090-1095.
(45.) Papakostas GI, Petersen T, Mischoulon D, et al. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 2: predictors of relapse during the continuation phase of pharmacotherapy. J Clin Psychiatry 2004;65:1096-1098.
(46.) Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol 2003;23:309-313.
(47.) Alpert JE, Mischoulon D, Rubenstein GE, et al. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry 2002;14:33-38.
(48.) Passeri M, Cucinotta D, Abate G, et al. Oral 5'-methyltetrahydrofolic acid in senile organic mental disorders with depression: results of a double-blind multicenter study. Aging (Milano) 1993;5:63-71.
(49.) Godfrey PS, Toone BK, Carney MW, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990;336:392-395.
(50.) Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord 2000;60:121-130.
(51.) Coppen A, Chaudhry S, Swade C. Folic acid enhances lithium prophylaxis. J Affect Disord 1986;10:9-13.
(52.) Botez MI, Peyronnard JM, Berube L, Labrecque R. Relapsing neuropathy, cerebral atrophy and folate deficiency. A close association. Appl Neurophysiol 1979;42:171-183.
(53.) Vogel RI, Fink RA, Schneider LC, et al. The effect of folic acid on gingival health. J Periodontol 1976;47:667-668.
(54.) Thomson ME, Pack AR. Effects of extended systemic and topical folate supplementation on gingivitis of pregnancy. J Clin Periodontol 1982;9:275-280.
(55.) Pack AR. Folate mouthwash: effects on established gingivitis in periodontal patients. J Clin Periodontol 1984;11:619-628.
(56.) No authors listed. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991;338:131-137.
(57.) Vergel RG, Sanchez LR, Heredero BL, et al. Primary prevention of neural tube defects with folic acid supplementation: Cuban experience. Prenat Diagn 1990;10:149-152.
(58.) Milunsky A, Jick H, Jick SS, et al. Multivitamin/ folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989;262:2847-2852.
(59.) Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832-1835.
(60.) Bower C, Stanley FJ. Dietary folate as a risk factor for neural tube defects: evidence from a case-control study in Western Australia. Med J Aust 1989;150:613-619.
(61.) Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993;269:1257-1261.
(62.) Shaw GM, Schaffer D, Velie EM, et al. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology 1995;6:219-226.
(63.) Tamura T, Goldenberg RL, Freeberg LE, et al. Maternal serum folate and zinc concentrations and their relationships to pregnancy outcome. Am J Clin Nutr 1992;56:365-370.
(64.) Scholl TO, Hediger ML, Schall JI, et al. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr 1996;63:520-525.
(65.) Frelut ML, de Courcy GP, Christides JP, et al. Relationship between maternal folate status and foetal hypotrophy in a population with a good socio-economical level. Int J Vitam Nutr Res 1995;65:267-271.
(66.) Montes LF, Diaz ML, Lajous J, Garcia NJ. Folic acid and vitamin B12 in vitiligo: a nutritional approach. Cutis 1992;50:39-42.
(67.) Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol 1997;77:460-462.
(68.) Russell RM, Golner BB, Krasinski SD, et al. Effect of antacid and H2 receptor antagonists on the intestinal absorption of folic acid. J Lab Clin Med 1988;112:458-463.
(69.) Lambie DG, Johnson RH. Drugs and folate metabolism. Drugs 1985;30:145-155.
(70.) Pironi L, Cornia GL, Ursitti MA, et al. Evaluation of oral administration of folic and folinic acid to prevent folate deficiency in patients with inflammatory bowel disease treated with salicylazosulfapyridine. Int J Clin Pharmacol Res 1988;8:143-148.
(71.) Backman N, Holm AK, Hanstrom L, et al. Folate treatment of diphenylhydantoin-induced gingival hyperplasia. Scand J Dent Res 1989;97:222-232.
(72.) van Ede AE, Laan RF, Rood M J, et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2001;44:1515-1524.
(73.) Gori T, Burstein JM, Ahmed S, et al. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation 2001;104:1119-1123.
(74.) Butterworth CE Jr, Tamura T. Folic acid safety and toxicity: a brief review. Am J Clin Nutr 1989;50:353-358.
(75.) Goggin T, Gough H, Bissessar A, et al. A comparative study of the relative effects of anticonvulsant drugs and dietary folate on the red cell folate status of patients with epilepsy. Q J Med 1987;65:911-919.
COPYRIGHT 2005 Thorne Research Inc.
COPYRIGHT 2005 Gale Group