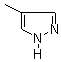
Ethylene glycol, a solvent found in antifreeze, may result in metabolic acidosis, renal failure, nervous system damage and even death when ingested by humans. Standard therapy includes high dosages of oral or intravenous ethanol and hemodialysis. Ethanol inhibits alcohol dehydrogenase, but because it must be used at intoxicating dosages, it may cause hepatotoxicity and hypoglycemia. Fomepizole (4- methylpyrazole) is an inhibitor of alcohol dehydrogenase but does not appear to have the adverse effects of ethanol. Brent and associates studied the efficacy of fomepizole in the treatment of ethylene glycol poisoning.
Twenty-three patients at least 12 years of age who presented with confirmed or suspected ethylene glycol poisoning were enrolled in the study. Criteria for poisoning included one of three characteristics: (1) a plasma ethylene glycol level of at least 20 mg per dL (3 mmol per L); (2) suspected ingestion of ethylene glycol along with three of four laboratory findings, including an arterial pH of less than 7.3, a bicarbonate level of less than 20 mEq per L, an osmolar gap of greater than 10 mOsm per L and oxaluria; (3) suspected ingestion of ethylene glycol within the preceding hour and a serum osmolar gap of greater than 10 mOsm per L.
Patients were given intravenous fomepizole, in a loading dose of 15 mg per kg of body weight followed by 10 mg per kg every 12 hours for 48 hours. This regimen was followed by a repeat dosing of 15 mg per kg every 12 hours to compensate for increased fomepizole metabolism. The patients were treated until their ethylene glycol level was less than 20 mg per dL.
After the loading dose of fomepizole, patients underwent hemodialysis if the initial ethylene glycol level was 50 mg per dL (8 mmol per L) or greater, if the arterial pH dropped below 7.1, if the arterial pH dropped below 7.3 despite intravenous bicarbonate, if the serum creatinine level increased by at least 1 mg per dL (88 mmol per L) above baseline or if the creatinine level was more than 3 mg per dL (265 mmol per L). Continuous cardiac monitoring, assessment of urinary output and frequent laboratory studies were performed in all patients. The main end points of the study were development of renal injury, additional production of ethylene glycol metabolites (including urinary excretion of oxalate) and the development of cranial neuropathies.
After initial enrollment, four of the 23 patients were found to have plasma ethylene glycol concentrations less than 20 mg per dL or did not meet other criteria for entry and were excluded from the study. Of the remaining 19 patients, 14 were determined to have ingested antifreeze as the source of the ethylene glycol. On presentation, seven patients were comatose, seven were awake, three were inebriated and two were lethargic. Based on the previously noted criteria, 17 patients required hemodialysis. The average number of doses of fomepizole was 3.5, with a range of one to seven. The plasma glycolate levels decreased progressively in all patients, and the pH and bicarbonate levels concurrently increased. None of the patients showed deterioration in mental status from baseline, and there were no episodes of hypoglycemia.
Concerning other outcomes, 18 of the 19 patients survived. The patient who died suffered a myocardial infarction before enrollment and had a baseline pH of 7.05. There were no cases of cranial neuropathy. Ten patients had a normal creatinine level at entry, and none sustained renal injury during treatment with fomepizole. Of the nine patients who had an elevated creatinine level at baseline, all showed worsening renal function during treatment. However, the creatinine level normalized in six of the patients and in the other three, it ranged from 1.5 to 3.8 mg per dL (132 to 335 mmol per L) at the time of discharge. All patients with renal injury had initial ethylene glycol levels of 98 mg per dL (16 mmol per L) or greater. The only adverse events possibly related to treatment with fomepizole were transient bradycardia (heart rate of 50 to 60 beats per minute) in two patients, headaches in two patients and brief seizures that occurred 15 minutes after the first dose of medication but not with subsequent dosing in two patients.
The authors conclude that fomepizole is a safe and effective treatment for patients who have ingested ethylene glycol. When given early, it prevents renal injury and does not appear to cause hypoglycemia or mental status changes, problems that are seen when ethanol is used for treatment.
Jeffrey T. Kirchner, D.O.
Brent J, et al. Fomepizole for the treatment of ethylene glycol poisoning. N Engl J Med March 18, 1999; 340:832-8.
COPYRIGHT 1999 American Academy of Family Physicians
COPYRIGHT 2000 Gale Group