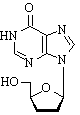
Hydroxurea is a popular, but unlicensed, means of intensifying treatment in patients receiving didanosine, an inhibitor of nucleoside reverse transcriptase (a "nucleoside analogue") used in combination with other retroviral drugs for the treatment of HIV infection. We report on a patient who developed pancreatitis after hydroxyurea was added to didanosine.
In January 1998 a 26 year old man who was HIV positive started taking stavudine 40 mg twice daily (Zerit, Bristol-Myers Squibb), didanosine 400 mg daily (Videx, Bristol-Myers Squibb), and nevirapine 200 mg twice daily (Viramune, Boehringer Ingelheim) because of a falling CD4 count (250x [10.sup.6]/l), high viral load (81 747 copies/ml), and symptoms related to HIV. He was receiving no other treatment. He had no additional risk factors for pancreatitis. Response to treatment was good: the viral load decreased to undetectable levels, the CD4 count increased to 470x [10.sup.6]/l, and the symptoms improved, enabling the patient to resume full time employment. In June 1999 the viral load increased to 1390 copies/ml despite the patient's adherence to treatment, so treatment was intensified with hydroxyurea 500 mg twice daily (Hydrea, Bristol-Myers Squibb). The viral load decreased to 237 copies/ml. The patient began to experience malaise and pain in the upper abdomen. This was attributed to the hydroxyurea, which was stopped after 42 days. The symptoms worsened, and three weeks later he was admitted to hospital with severe pain, vomiting, fever, tenderness of the upper abdomen, and guarding. Amylase concentration was 746 units (normal range [is less than] 300), with neutrophil leucocytosis (18.1x[10.sup.9]/l). Computed tomography showed changes consistent with pancreatitis. All drugs were stopped. The patient made an uneventful recovery with conservative treatment. He is no longer taking antiretroviral drugs.
Hydroxyurea potentiates the action' of nucleoside analogues by depletion of the deoxyribonucleoside triphosphate pool, particularly deoxyadenosine triphosphate.[1] This increases viral uptake of nudeoside analogues, especially didanosine, which competes against deoxyadenosine triphosphate. Few side effects have been reported with hydroxyurea used in conjunction with didanosine.[1] Pancreatitis is a dose dependent adverse event of didanosine, occurring in 0.7-5.5% of patients receiving doses between 200 mg and 750 mg per day, almost always within the first nine months,[2-3] Thus, adding hydroxyurea to our patient's treatment may have precipitated didanosine induced pancreatitis, perhaps by potentiating the intracellular toxicity of didanosine. Precipitating serious side effects of previously well tolerated drugs is a newly recognised hazard of hydroxyurea.
This adverse event has been reported to the Medicines Control Agency and Bristol-Myers Squibb. It has not been reported previously.
Competing interests: None declared.
[1] Lori F, Malykh AG, Foli A, Maserati R, De Antoni A, Minolo L, et al. Combination of a drug targeting the cell with a drug targeting the virus controls human immunodeficiency virus type 1 resistance. AIDS Res Human Retroviruses 1997;13:1403-9.
[2] Alpha International Coordinating Committee. The alpha trial: European/Australian randomised double-blind trial of two doses of didanosine in zidovudine-intolerant patients with symptomatic HIV disease. AIDS 1996; 10:867-80.
[3] Bristol Myers Squibb. Health Care Provider Letter. Princeton, Nov 1999.
HJ Longhurst, AJ Pinching, St Bartholomew's Hospital, London EC1A 7BE
COPYRIGHT 2001 British Medical Association
COPYRIGHT 2001 Gale Group