Chlorpyrifos targets mammalian brain development through a combination of effects directed at cholinergic receptors and intracellular signaling cascades that are involved in cell differentiation. We used sea urchin embryos as an invertebrate model system to explore the cellular mechanisms underlying the actions of chlorpyrifos and to delineate the critical period of developmental vulnerability. Sea urchin embryos and larvae were exposed to chlorpyrifos at different stages of development ranging from early cell cleavages through the prism stage. Although early cleavages were unaffected even at high chlorpyrifos concentrations, micromolar concentrations added at the mid-blastula stage evoked a prominent change in cell phenotype and overall larval structure, with appearance of pigmented cells followed by their accumulation in an extralarval cap that was extruded from the animal pole. At higher concentrations (20-40 [micro]M), these abnormal cells constituted over 90% of the total cell number. Studies with cholinergic receptor blocking agents and protein kinase C inhibitors indicated two distinct types of effects, one mediated through stimulation of nicotinic cholinergic receptors and the other targeting intracellular signaling. The effects of chlorpyrifos were not mimicked by chlorpyrifos oxon, the active metabolite that inhibits cholinesterase, nor by nonorganophosphate cholinesterase inhibitors. Dieldrin, an organochlorine that targets [GABA.sub.A] receptors, was similarly ineffective. The effects of chlorpyrifos and its underlying cholinergic and signaling-related mechanisms parallel prior findings in mammalian embryonic central nervous system. Invertebrate test systems may thus provide both a screening procedure for potential neuroteratogenesis by organophosphate-related compounds, as well as a system with which to uncover novel mechanisms underlying developmental vulnerability. Key words: acetylcholine, chlorpyrifos, cholinergic receptors, development, dieldrin, GABA, neurotoxicity, organophosphate pesticides, sea urchin. Environ Health Perspect 109:651-661 (2001). [Online 20 June 2001]
http://ehpnet1.niehs.nih.gov/docs/2001/109p651-661buznikov/abstract .html
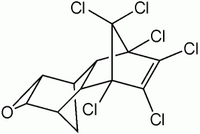
The adverse environmental impact of organochlorine pesticides has led to their gradual replacement with less damaging alternatives, notably the organophosphates. Chlorpyrifos (O, O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate) is one of the most widely used organophosphates, largely because of its stability and persistence. Recently, however, a new concern has arisen about exposure of pregnant women and children (1-3), and in the United States limitations have been placed on domestic use, where exposures may be particularly high (4-7). Its major use in agriculture or other professional applications remains unabated throughout the world and represents a growing dilemma for storage and disposal.
The acute systemic toxicity of organophosphates reflects cholinergic hyperstimulation due to inhibition of cholinesterase; in the case of chlorpyrifos, this action is produced by its active metabolite, chlorpyrifos oxon. However, it is increasingly clear that the developmental neurotoxicity of chlorpyrifos comprises other actions of the native compound itself (2,3,8-16). In vivo studies with developing rodents have identified several processes targeted by chlorpyrifos, including neural cell replication (8,11), neurite outgrowth (16), signaling cascades and transcriptional events involved in neural cell differentiation (9,12), oxidative stress (14,17,18), and cell damage and apoptosis (19,20), all of which culminate in altered synaptic neurotransmission (21-25) and consequent behavioral anomalies (13,24). Indeed, both chlorpyrifos and chlorpyrifos oxon are capable of direct actions on muscarinic and nicotinic cholinergic receptors, influencing second messenger production (26-28) and ion fluxes (29,30); hence, even some of the cholinotypic actions on developing systems may be mechanistically unrelated to inhibition of cholinesterase activity.
The problems inherent with in vivo treatments--such as maternal toxicity, alterations in the physiology of the maternal-fetal unit, hormonal imbalances, or changes in metabolism and nutrition--have rendered it difficult to identify the actual cellular targets of chlorpyrifos in the developing brain. Consequently, attempts have been made to model the effects in vitro, using either transformed cell lines with neurotypic features (11,12,14,16-18,31-33) or cultures of immature brain tissue (15,19,34). These approaches share a different set of limitations. Transformed cell lines are not identical to neural cells and are not only typically less responsive to neurotoxins such as chlorpyrifos (11) but, as transformed cells, have abnormal replication and differentiation patterns. Furthermore, a single cell line cannot recapitulate the cell-to-cell signaling events that are likely to be targeted by chlorpyrifos in the developing brain in vivo, most notably cholinergic and monoaminergic neurotransmission (2,3,21-23,35) and transduction components such as the protein kinases that are linked to these transmitters (9,26-28,36). Aggregating cultures of mixed neuronal and glial cells (15), micromass cultures from fetal brain regions (34), or cultured embryos (19) may obviate the problem of single-cell cultures, but are limited in their applicability only to short periods of development, whereas the actions of chlorpyrifos are likely to extend over a broad temporal range (1-3,37-40).
It would thus be extremely worthwhile to develop a model system that encompasses development across multiple stages and that requires cell-to-cell communication specifically involving cholinergic and monoaminergic neurotransmitters and their signaling cascades. Ideally, the system could then be used to identify specific cellular mechanisms for the actions of chlorpyrifos, notably the cholinergic receptors and appropriate signaling cascades, as well as potentially providing a screening mechanism with which to compare chlorpyrifos to other organophosphates or alternative classes of pesticides.
In the current study, we have explored the sea urchin embryo and larva as a test system. In the course of ontogeny, the sea urchin, like the mammalian central nervous system (CNS), develops a functional cholinergic response system, comprising the requisite receptors and signaling cascades, with expression of these components evolving over a defined developmental period surrounding cell differentiation and morphological assembly (41,42). Thus, events involved in neuroteratogenicity in the mammalian brain are paralleled by "pre-nervous" morphogenetic anomalies in the developing sea urchin (42-48). Importantly, sea urchin embryos are unaffected by cholinesterase inhibition per se; thus, in them we can examine developmental mechanisms, including those involving cholinergic systems, that are distinct from those associated with anti-cholinesterase actions, the effects that underlie the systemic toxicity of organophosphates in mammals (41,44,47,49). Additionally, the normal morphologic and biochemical features of sea urchin embryonic and larval development are well characterized, as are the classes of developmental malformations evoked by a wide variety of embryotoxins and teratogens (43,46,50,51), so the effects of chlorpyrifos or other pesticides can be compared mechanistically to compounds with defined mechanisms of action.
Besides using the sea urchin to identify the developmental processes affected by chlorpyrifos exposure, we have characterized the temporal events that define the critical period of vulnerability and, by identifying specific cell signaling targets, have performed a preliminary evaluation of potential antidotes that might prevent developmental toxicity. We also contrasted the effects of chlorpyrifos with those of the organochlorine pesticide dieldrin, which targets [GABA.sup.A] receptors (52,53), to determine whether the actions of the organophosphate represents selective effects, as opposed to more general developmental toxicity shared by other pesticide classes.
Materials and Methods
Sea urchins Strongylocentrotus droebachiensis and S. purpuratus were trawled or captured using SCUBA near Friday Harbor, Washington, USA. They were maintained up to 4-6 months with their normal food (kelp) in aquaria with continuously flowing sea water (8-10 [degrees] C) at Friday Harbor Laboratories. The techniques of insemination and handling of embryos and larvae were conducted as reported previously (51). We obtained the eggs or sperm by injecting the ripe animals with 0.8-1.0 mL (S. droebachiensis) or 0.3-0.5 mL (S. purpuratus) of 0.55 M KCl. Some animals were re-injected after about 20 days. Artificial sea water (ASW; 445 mM NaCl, 24.6 mM Mg[Cl.sub.2], 18.1 mM Mg[SO.sub.4], 9.2 mM KCl, 2.36 mM Na[HCO.sub.3], 11.5 mM Ca[Cl.sub.2]; pH 7.5) was used as the incubation medium. The suspensions of unfertilized eggs were passed through a nylon mesh for elimination of the jelly coat.
We obtained embryos and larvae by fertilizing eggs from 19 females of S. droebachiensis (28 batches) and 9 females of S. purpuratus (14 batches) and placed them in multiwell cell culture plates. Test substances were introduced into the wells approximately 30 min after the insemination of the eggs (one-cell stage) or later, at the mid-blastula, late (mesenchyme) blastula, gastrula, or prism stages. Suspensions varied from 40 to 60 (S. droebachiensis) or 100 to 150 (S. purpuratus) per mL and were approximately the same for all treatments and across experiments. Embryos and larvae in control wells were incubated in pure ASW and in ASW with corresponding quantities of vehicles (distilled water, methanol, or ethanol--see below). The final incubation volume was 1 or 2 mL and the temperature was maintained at approximately 10 [degrees] C. Embryonic and larval development were viewed in a microscope and videotaped using a video camera. Images were transferred to a video cassette recorder and then digitized. The videotaping was sometimes performed throughout an experiment at intervals of 4-24 hr or, more often, at the end of an experiment, when the treated embryos or larvae displayed maximally abnormal phenotypes. Initially all embryos or larvae in a given well were videotaped at low (40x) magnification (Figure 1). For nearly every treatment paradigm, all (100%) specimens showed the same phenotype, so representative specimens were imaged at higher magnification (100x or 200x) for presentation.
[ILLUSTRATIONS OMITTED]
The following substances were tested (the concentration of stock solution and vehicle used are given in parentheses): dieldrin (40 mM in ethanol or DMSO) and chlorpyrifos (20 mM in methanol) (both from Chem Service, West Chester, PA, USA); chlorpyrifos oxon and 3,5,6-trichloropyridinol (both 40 mM in methanol) supplied by the U.S. Environmental Protection Agency (Research Triangle Park, NC, USA); imechine (Latoxan, Valence, France), a hydrophilic antagonist of neuronal nicotinic acetylcholine receptors (nAChR), and the noncompetitive antagonist QX-222 (Astra Pharmaceuticals, Westborough, MA, USA) (both at 20 mM in distilled water); atropine sulfate (RBI, Natick, MA, USA), a muscarinic acetylcholine receptor antagonist (40 mM in distilled water); 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H-7), an inhibitor of protein kinase (PK) C, and N-(2-guanidinoethyl)-5-isoquinolinesulfonamide (HA-1004), an inhibitor of PKA (both from RBI, and each at 20 mM in distilled water).
In addition, we tested two novel derivatives of polyunsaturated fatty acids: dimethylaminoethyl esters of arachidonic and docosahexaenoic acids (AA-DMAE, 26.6 mM, and DHA-DMAE, 25 mM in ethanol). These new compounds (Figure 2) were synthesized in the Laboratory of Oxylipins, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Moscow (54,55). Based on our recent results (55,56), AA-DMAE and DHA-DMAE act on sea urchin embryos and larvae as antagonists of acetylcholine receptors. Because of their high lipophilicity, these substances penetrate the cytoplasm of embryonic or larval cells to act on both intracellular and cell surface receptors. This is potentially critical to the current model because the coexistence of intracellular and surface-located transmitter receptors is typical for sea urchin embryos and early larvae (45).
[ILLUSTRATION OMITTED]
The final concentrations of all substances were prepared in ASW. Vehicle-treated embryos and larvae developed completely normally up to the end of the observation period at the onset of active feeding; the highest final concentrations of vehicles were 0.2-0.4% by volume. All comparisons show chlorpyrifos- or drug-exposed embryos and larvae juxtaposed to the comparable, vehicle-treated control group.
In additional experiments to assess the stability of chlorpyrifos oxon in ASW, we prepared chlorpyrifos oxon as a 40 mM stock solution in methanol and then added it to ASW to achieve a final concentration of 40 [micro]M, which was incubated for 24 hr at 10 [degrees] C. Aliquots were taken immediately after addition of the chlorpyrifos-oxon, and after 3 and 24 hr of incubation. Extractions and analysis were performed as described earlier (57).
Results
Effects of dieldrin and chlorpyrifos. In 14 experimental series on S. droebachiensis (14 batches of eggs obtained from 9 females) and 6 series on S. purpuratus (6 batches from 6 females), dieldrin at all concentrations (10-200 [micro]M) had little or no effect on embryonic or larval development up to the start of active feeding, at which point observations were discontinued (Figure 3). Similar, negative results were obtained when dieldrin was introduced at any stage of development from one-cell up to the prism stage. Judged by standard larval behavioral criteria, e.g., swimming activity, the nervous system of the dieldrin-treated larvae appeared to function normally. At the highest nominal concentrations of dieldrin (100-200 [micro]M, its actual concentration in ASW may be limited by its low solubility.
[ILLUSTRATIONS OMITTED]
In contrast to dieldrin, chlorpyrifos evoked marked concentration- and stage-dependent developmental anomalies, identical for both sea urchin species (22 batches from 16 females of S. droebachiensis and 11 batches from 7 females of S. purpuratus). A summary of the results, along with the numbers of embryos and larvae for each treatment paradigm appears in Tables 1 and 2. However, because the embryos of S. purpuratus are smaller and highly pigmented, we present pictures only of representative embryos and larvae of S. droebachiensis in the figures illustrating the defects (Figures 4-10).
[ILLUSTRATIONS OMITTED]
We evaluated three different treatment paradigms (Tables 1 and 2): chlorpyrifos added 30 min after fertilization, added at the mid-blastula 2 to late blastula 1 stage (24-30 hr after fertilization), or added at gastrulation (43-46 hr after fertilization). Evaluations were then conducted at the mid-blastula 1 stage, late blastula 1 or 2 through early gastrula 1 stage, late gastrula-early prism stage, or prism stage; the characteristics of each stage have been described previously (51). When we added chlorpyrifos 30 min after fertilization and examined embryos at the mid-blastula 1 stage (22 hr after fertilization), we did not observe any defects, even at very high concentrations (160 [micro]M). When we examined these same embryos 10 hr later, at the late blastula 1 or 2 stage, every embryo exposed at a concentration of 15 [micro]M or higher displayed developmental anomalies, with the type of defect intensifying as the concentration was raised. Accordingly, we next examined a later exposure paradigm (chlorpyrifos added 24-30 hr after fertilization) begun at mid-blastula 2 through late blastula 1--i.e., from the last stages of embryonic development (when embryos begin to rotate inside the fertilization envelope because of ciliary beating, and are hatching) to the first larval stage. This later exposure period exhibited the maximum sensitivity to chlorpyrifos, with anomalies present at only 1-2 [micro]M. Sensitivity then declined, so exposure begun 46 hr after fertilization (during gastrulation) was less effective. Therefore, for evaluations of the concentration threshold, or for interactions with blocking agents or metabolic inhibitors, we performed most studies using exposure begun at the mid-blastula 2 to late blastula 1 stage. Because we obtained a high incidence of anomalies (75%) at 1-2 [micro]M chlorpyrifos, we performed preliminary experiments on S. droebachiensis and found some anomalies at even lower concentrations (between 0.5 and 1 [micro]M), similar to thresholds seen in mammalian cell cultures or embryos (11,12,14, 16,19,34). At slightly higher concentrations (2.5-5 [micro]M and above), every one of the thousands of embryos or larvae receiving chlorpyrifos treatment displayed developmental anomalies (Tables 1 and 2, Figure 1); the morphological characteristics of each type of anomaly are described below.
After introduction of 20-40 [micro]M chlorpyrifos during the period of maximum sensitivity (late blastula 1 stage, 30 hr after fertilization) anomalies became evident by the early gastrula stage, 5-6 hr after addition of chlorpyrifos (Figure 4). Beginning at that time, the number of mesenchyme cells migrating into the blastocele was increased above normal levels. These cells then started to darken, losing their transparency (Figure 4A, B), and became located along the inner surface of the body wall; in contrast, cells in normal larvae retained their transparency and were located near the primary gut (archenteron), moving later through the blastocele to the animal pole (Figure 4C, F).
One or 2 hr later, the darkened cells of chlorpyrifos-exposed larvae began to show extrusion from the animal pole (extrusion, not proliferation--see below). During the next 2-3 hr the number of extruded cells increased sharply. We conducted preliminary estimates of the proportions of cells affected by measuring the relative area of extralarval cell conglomerates for all larvae treated with a given concentration of chlorpyrifos. Cell sizes in the conglomerates appeared to be similar to those in nontransformed parts of the larvae. Using this estimation, approximately 50% or more of all larval cells were extruded, maintaining, as earlier, their dark pigmentation (Figure 4D, E). Over the subsequent few hours, the number of extruded cells continued to rise, eventually comprising up to 80-90% of the total (Figure 4G, H). In contrast, there were never any signs of cell extrusion during the development of control larvae (Figure 4F, I).
As a result of chlorpyrifos treatment in this sensitive period, a compact extralarval cap, largely comprised of pigmented cells, formed at the animal pole, and the blastocele, indeed the larva itself, was correspondingly diminished because of both cell extrusion and compaction. The size of the caps of these mushroom-shaped larvae showed a direct correspondence to the chlorpyrifos concentration. At high concentrations (40-80 [micro]M), the cap often enveloped nearly the entire larva and contained more than 90% of total cells (Figure 5A), resulting in death within a few hours (defined as a maximal defect, category IV in Tables 1 and 2). At 20-30 [micro]M (Figures 4 and 6), the larvae remained motile, retained the blastopore and the lower part of archenteron and generally survived through the next 1-2 days; they did not lose their extralarval caps (category III). With a further reduction to 15 [micro]M chlorpyrifos, the fully developed caps were smaller, comprising only 40-60% of all cells, and were round in appearance, delimited very clearly from the larva itself (Figure 6B). In this case, the caps sometimes were lost because of ciliary beating, and larvae that lost their caps continued to develop for the ensuing 1-2 days; however, we did not follow their ultimate fate. Regardless of whether they retained or lost the cap, most of the chlorpyrifos-exposed larvae began to exogastrulate and thus became nonviable (category II).
With a further decrement in the concentration of chlorpyrifos (5-10 [micro]M) we observed the formation of much smaller extralarval caps (see Figure 10A, below). Typically, these caps were lost during development and hence the larvae retained some viability (a variant of category II). Accordingly, with this treatment group, we were able to delineate the anatomical features of the extralarval cell caps. Before its loss, the cap was connected to the animal pole of the larva by a thin stalk. In fact, the whole animal surface of the larva was intact and covered by cilia after the loss of the cap, implying that the cap was really an extralarval abnormal structure, not part of the larva itself.
At even lower chlorpyrifos concentrations (2.5-5 [micro]M), there was no extrusion of cells from the blastocele. Instead, the pigmented cells that arose 5-6 hr after addition of chlorpyrifos accumulated inside the larva (Figure 5B), rather than in an external cap. Later, these larvae also began to exogastrulate (category I, seen near the threshold concentration). This defect thus culminated in a different structural outcome, despite the fact that the same type of cellular anomaly was obtained as at the higher concentrations. Lower concentrations of chlorpyrifos did not perturb larval development up to the beginning of active feeding (category 0, no effect). The types of defects and their concentration dependence were highly reproducible across different batches of embryos and larvae and across the two species examined (Tables 1 and 2).
Up to this point, the description of defects pertains to those observed after exposure was begun during the developmental stages most sensitive to chlorpyrifos (mid-blastula 2 to late blastula 1). These categories of defects can also be used to describe responses elicited during the less-sensitive periods preceding and following the sensitive period. Adding chlorpyrifos at the one-cell stage, even at the highest concentrations (40-160 [micro]M), had no initial effect, and abnormalities did not appear until the mid-blastula stage (Figure 7A, Tables 1 and 2). Some of these embryos remained normal at the mid-blastula 2 stage (category 0), whereas others exhibited anomalies: inhibition of hatching, precocious appearance of mesenchyme cells in the blastocele, and extrusion of some cells to the perivitelline space (Figure 7C, 8A). These defects (ranging across all four categories) were concentration-dependent (Tables 1 and 2); for example, the number of cells extruded into the perivitelline space varied for different chlorpyrifos concentration from single (category I) or few (category II, Figure 7C) to half or a majority of cells (categories III-IV, Figure 8A). In the embryos that hatched, cell extrusion and cap-like malformations were evident, incorporating most larval cells, as described earlier (Figure 7E). The caps were clearly delimited from the larvae (compare Figure 7E to Figure 6A or 9C) and were usually lost at the time that control larvae reached the prism stage. The blastopore and archenteron were visible in the diminished vegetal half of chlorpyrifos-treated larvae. There were no exogastrulae with this treatment regimen, so these embryos were capable of normal gastrulation. In the embryo population that failed to hatch (comprising all the embryos when the chlorpyrifos concentration was raised to 80-160 [micro]M), extrusion of abnormal cells continued into the perivitelline space (Figure 8A, category IV). These cells did not form the compact cap and were instead suspended in the perivitelline fluid, moving inside the fertilization membrane with rotation of the blastula. The embryos of the nonhatching population were unable to achieve gastrulation and thus were ultimately nonviable.
In keeping with the concept that initial stages of development are relatively unaffected by chlorpyrifos treatment, the threshold concentration for adverse effects added immediately after fertilization ([is greater than] 10 [micro]M) was higher than that described above for exposure during subsequent developmental stages. However, we also obtained evidence for closure of the critical period for the effects of chlorpyrifos: The sensitivity began to decrease simultaneously with gastrulation; the threshold concentration necessary to elicit anomalies rose sequentially to more than 10 [micro]M (Tables 1 and 2). When chlorpyrifos was introduced at the mid or late gastrula stage, the extrusion of cells started almost immediately during the next 1-2 hr. At this stage, the extruded cells were semitransparent and did not form a compact cap. Rather, they assembled into loose clusters to form a plume-like shape (Figure 8C), comprising a much smaller proportion (~ 15-30%) of total larval cells. These larvae were nonviable at 80 [micro]M chlorpyrifos (category IV in this group of experiments) or, at slightly lower concentrations (40 [micro]M, category III), survived for the next 1-3 days (Tables 1 and 2). Cells of the latter group were shed continuously and the plume finally became separated from the motile larva, leaving behind an unusually pigmented animal part, considerably broadened compared to the transparent vegetative part. By decreasing the chlorpyrifos concentration further, to 15-20 [micro]M (Tables 1 and 2), we obtained larvae with a smaller plume (category II) or with cells accumulated inside the blastocele without extrusion (category I).
Finally, chlorpyrifos, added at the higher threshold required for effects at the prism stage or later (~15-20 [micro]M), did not evoke cell extrusion, but nevertheless continued to kill larvae, indicating additional toxic mechanisms unrelated to the types of developmental anomalies described for earlier stages. We have not yet evaluated the potential morphological defects accompanying this late stage of chlorpyrifos exposure.
Chlorpyrifos metabolites. We conducted additional studies (6 series on S. droebachiensis and 3 series on S. purpuratus) to test the actions of chlorpyrifos metabolites: chlorpyrifos oxon, the active metabolite that inhibits cholinesterase and is thus responsible for the acute toxicity of chlorpyrifos, and 3,5,6-trichloropyridinol, the main catabolic product of chlorpyrifos. Neither of these metabolites (40-80 [micro]M), added immediately after fertilization or at the mid-blastula 2 stage (when the sensitivity to chlorpyrifos was the highest), evoked any developmental anomalies (Figure 9). To ensure that the negative findings for chlorpyrifos oxon did not reflect chemical degradation of the compound in ASW, we evaluated its stability under our incubation conditions. High-performance liquid chromatographic analysis (57) of 40 mM chlorpyrifos oxon incubated with ASW for up to 24 hr indicated absolutely no breakdown for at least 24 hr (no further measures were taken).
Antagonism of the effects of chlorpyrifos. If chlorpyrifos elicits developmental anomalies through effects on cholinergic receptors or signaling cascades linked to the receptors, then antagonists should prevent or augment the effects. The hydrophilic nAChR antagonists imechine and QX-222 (25-50 [micro]M) produced partial protection from the adverse effects of chlorpyrifos, whereas lipophilic antagonists capable of penetrating the cell membrane, AA-DMAE or DHA-DMAE (20-40 [micro]M), reduced or prevented all the developmental anomalies (Figures 5, 6, 8). In additional studies, we found that these antagonists protected the late larvae (prism and pluteus stages) from chlorpyrifos-induced lethality. The muscarinic acetylcholine receptor antagonist atropine (10-100 [micro]M) did not change the sensitivity of sea urchin larvae to chlorpyrifos (experiments on S. droebachiensis only; data not shown).
We next evaluated inhibitors acting on signaling cascades downstream from the receptors. HA-1004, a PKA inhibitor, did not influence the adverse effects of chlorpyrifos (Figure 10) but H-7, a PKC inhibitor, potentiated chlorpyrifos' actions. In the presence of H-7 (10-20 [micro]M), 10 [micro]M chlorpyrifos elicited actions approximating those seen with a higher concentration (40 [micro]M) of chlorpyrifos alone (Figure 10B, compared to Figure 5A). Although these concentrations of H-7 had no effect on the embryos by themselves (Figure 10D), they are fully effective in preventing teratogenic actions of phorbol 12-myristate 13-acetate and other PKC activators (47).
[ILLUSTRATIONS OMITTED]
We observed a similar pattern when chlorpyrifos was added after fertilization. AA-DMAE and DHA-DMAE protected the embryos, even if they were added at the early blastula 2 to mid-blastula 1 stages, 10-12 hr after chlorpyrifos. HA-1004 did not change the sensitivity to chlorpyrifos when the latter compound was added after fertilization, whereas again H-7 increased the sensitivity. In these results, as in the studies with chlorpyrifos alone, sea urchin embryos were relatively insensitive during cleavage divisions and blastulation, but displayed high sensitivity at the mid-blastula 2 stage.
Discussion
The present results indicate that sea urchin embryos and larvae represent a promising invertebrate model system for the evaluation of developmental neurotoxicity of organophosphate pesticides, with regard to both screening procedures and mechanistic investigations. Sea urchins produce numerous offspring that develop rapidly and with a specified sequence of morphological events that are readily apparent with routine microscopy. Adverse effects as seen here occur in a nearly all-or-none manner, with a very sharp demarcation between normal and abnormal embryos. Importantly, just like the mammalian CNS, the sea urchin expresses nAChRs that are linked to cell replication, differentiation, and apoptosis; so in this species, xenobiotic effects on cholinergic signaling are reflected by easily observable dysmorphogenesis, whereas comparable effects in the developing brain tend to elicit more subtle alterations that entail biochemical and functional assessments (1-3,39,42-47,49). In the case of chlorpyrifos, our findings for the underlying mechanisms and critical period of vulnerability in sea urchins are consistent, as discussed below, with the dual aspect of this pesticide's actions on mammalian CNS development, comprising both cholinergic and noncholinergic components (3,8,9,11,12,14,16,19).
In the developing mammalian brain, the vulnerability to chlorpyrifos emerges coincidentally with that of nAChR expression, so that at the neural tube stage, interference with mitosis and production of apoptosis show a specified progression with nAChR concentration (19,58). Subsequently, during postnatal development, there are at least two distinct effect classes. The first is independent of cholinergic receptor expression and influences cell replication through effects on intracellular messengers (9,12,14). With the progressive rise in receptor expression in cholinergically enriched regions (59), a second set of effects emerges, influencing cell replication and neuritic outgrowth, that is cholinergically related but nevertheless independent of the generation of chlorpyrifos oxon, the active metabolite that inhibits cholinesterase (3,8,10,16). These findings imply a direct interaction of chlorpyrifos with cholinergic receptors and signaling cascades in the developing mammalian CNS, consistent with results obtained from in vitro examinations of potential receptor targets (26,27,29).
With the present work in developing sea urchins, chlorpyrifos elicited developmental anomalies similar to those seen previously for cholinergic receptor agonists (55) such as nicotine (47). Hydrophilic nAChR antagonists that cannot penetrate the cell membrane (imechine, QX-222), but not the muscarinic acetylcholine receptor antagonist atropine, produced partial protection from chlorpyrifos treatment, implying that chlorpyrifos targets nAChRs, proteins that are readily found on the cell surface of sea urchin eggs, embryos, and larvae (45,47,60,61). Furthermore, the critical period of vulnerability to chlorpyrifos corresponded to the onset of gastrulation, coinciding with the beginning of neurotransmitter regulation of the developing cells and coordinated surges in acetylcholine and receptor levels (41,44,45,49). Thus, it is not just the presence or absence of the receptors that dictates the vulnerability to chlorpyrifos, but rather the cellular specification of receptor expression and the developmental context in which receptors are stimulated: Before gastrulation, neurotransmitters (including acetylcholine, serotonin, and other monoamines) and their receptors are expressed in all embryonic or larval cells (44,45). It is only with the rise in concentration of neurotransmitters and receptors, and their specialization into specific cell groups, that vulnerability to chlorpyrifos becomes apparent. Again, this finding parallels the stage-specific effects of chlorpyrifos on mammalian brain development (3,8). Finally, we were able to distinguish the relative importance of nAChRs as a potential target for developmental disruption by pesticides, since dieldrin, which targets [GABA.sub.A] receptors (62), had little or no effect.
There are two different potential mechanisms for the targeting of nAChRs by chlorpyrifos. If the organism produces chlorpyrifos oxon, leading to inhibition of cholinesterase, or if the chlorpyrifos concentration is high enough to cause enzymatic inhibition by itself (16), then endogenous cholinergic signals could be enhanced, producing inappropriate timing and intensity of trophic events linked to cholinergic receptors. However, this interpretation is entirely ruled out by our finding that chlorpyrifos oxon was totally ineffective in eliciting dysmorphogenesis. Indeed, prior work with nonorganophosphate cholinesterase inhibitors also failed to demonstrate interference with sea urchin embryonic and larval development (41,49), confirming the interpretation that cholinesterase inhibition per se is relatively unimportant in mediating the adverse effects of chlorpyrifos in this system. The second possibility is that chlorpyrifos itself interacts with nAChRs, eliciting cholinergic agonist-like effects. Indeed, both chlorpyrifos and its oxon interact with muscarinic cholinergic receptors as well as nAChRs and related ion channels (26-30). Thus, just like the developing mammalian CNS, chlorpyrifos targets cholinergic receptors in sea urchin embryos, leading to disruption of cellular differentiation and corresponding dysmorphogenesis, with a critical period delineated by emergence of nAChRs. Accordingly, chlorpyrifos evokes dysmorphogenesis through involvement of cholinergic mechanisms that are separable from inhibition of cholinesterase by itself or by its active metabolite, chlorpyrifos oxon.
Notwithstanding the involvement of membrane-associated nAChRs, chlorpyrifos clearly has additional actions contributing to altered development. Hydrophilic nAChR blocking agents (imechine, QX-222) caused only partial protection against the effects of chlorpyrifos, implying the existence of intracellular targets. In contrast, AA-DMAE and DHA-DMAE, which penetrate the cell membrane (55), provided complete protection from chlorpyrifos throughout all the sensitive developmental stages, including late larval stages (prisms and plutei). One possibility, then, is that chlorpyrifos targets intracellularly located nAChRs that may also play a role in cell differentiation. Previous evidence suggests that intracellular cholinergic receptors are present in sea urchin eggs and early embryos, and take part in triggering cleavage divisions (44,45). However, these early events lie outside the critical window for the adverse effects of chlorpyrifos observed here; we did not find any interference with cleavage divisions. Furthermore, if effects on nAChRs, whether on the cell surface or intracellularly, were the only effect of chlorpyrifos leading to dysmorphogenesis, then chlorpyrifos oxon should have been more effective, because it is at least as potent toward receptors and ion channels as chlorpyrifos itself (29,30).
The full spectrum of chlorpyrifos' effects may thus require the combination of nAChR targeting, plus other, intracellular events downstream from receptor activation. Previous work in the developing mammalian brain indicates that chlorpyrifos can interact with proteins involved in signaling cascades that transduce receptor signals (9,26-28). Certainly, the lipophilic moieties present on AA-DMAE and DHA-DMAE could extend activity toward other sites besides intracellular nAChRs, because the side chain of AA-DMAE is arachidonic acid, a potent intracellular messenger. In light of earlier work with mammalian brain that indicated effects of chlorpyrifos on PKA-related signaling cascades (9,26-28), we conducted studies of the interaction of chlorpyrifos with drugs known to interfere with two of the most prominent protein kinase-based signaling elements, PKA and PKC. We found that whereas a PKA inhibitor had no effect on the response to chlorpyrifos, a PKC inhibitor enhanced the ability of chlorpyrifos to disrupt embryonic and larval development. This suggests that at least one of the secondary sites of chlorpyrifos' actions is at the level of PKC activity. Again, this has a distinct parallel in mammalian brain. Inhibition of PKC during neurulation leads to dysmorphogenesis (63); this is also the stage for emergence of the effects of chlorpyrifos on mitosis and apoptosis. The concept that the net developmental effect of chlorpyrifos represents the interaction of targeting of PKC with effects mediated at the level of nAChRs is thus a novel area for future research. Indeed, this is precisely the point at which similar vulnerability emerges to nicotine during mammalian brain development, the most obvious nAChR-targeting prototype (58).
It is particularly interesting to note that we did not find any exacerbation of the effects of chlorpyrifos by a PKA inhibitor. Earlier work with the mammalian brain suggests that chlorpyrifos targets signaling cascades converging on the formation of cyclic AMP, including [G.sub.i]- and [G.sub.s]-linked receptors, G-proteins themselves, and adenylyl cyclase (9,26-28). It is obvious that this is not the case in the sea urchin model, implying either that any effects on signaling mediated through PKA occur "downstream" from primary effects on other cascades (e.g., PKC) or that development in the sea urchin does not provide an adequate model for this particular aspect of chlorpyrifos' actions on mammalian brain. Again, future work will be necessary to clarify this relationship.
In any case, the current results suggest that examination of the role of PKC in different stages of development of the mammalian CNS is warranted; indeed, recent work with phenobarbital and heroin suggests that neurobehavioral disruption by these agents also originates in changes in the expression and cholinergic responsiveness of PKC (64,65). The membrane-permeable cholinergic analogs described here, or chlorpyrifos itself, may thus provide important tools for dissecting the role of such intracellular mediators in neural cell differentiation. Our results with sea urchin embryos provide an initial glimpse into the role of cholinergic signaling and PKC in this process. The formation of an extralarval cell mass (mid-blastula stage) or cluster (late blastula and gastrula stages) was the most striking effect of chlorpyrifos. At the very least, the extralarval mass and cell extrusion both represent a major perturbation of morphogenesis, but are not likely to constitute simply an effect on cell proliferation or growth. Indeed, the extruded cells suspended in the perivitelline fluid or contained within the cap- or plume-like conglomerate were approximately the same size as normal larval cells at that stage of development. Given that the larva is not yet feeding, additional cells cannot be produced without a corresponding reduction in cell size, an effect that was not seen in the chlorpyrifos-treated group. Cell migration itself appears not to be an actual target for the effects of chlorpyrifos, because the movement of cells continued in the normal direction along the animal--vegetal axis, albeit that the cells were migrating into the enormous extralarval cell mass through an abnormal structure, the thin stalk attached to the animal pole. The reverse direction of cell movement (exogastrulation) was seen in some experimental situations, too, simultaneously with formation of the extralarval cap (see, for example, Figure 4C). Nevertheless, cells displaying altered morphology and location accumulated in the larva with or without subsequent extrusion (illustrated in Figure 5B, showing the effects of near-threshold chlorpyrifos concentrations, and Figure 9C, where there is broadening of the animal part of chlorpyrifos-treated larvae caused by transformed, pigmented cells), so altered differentiation was not inextricably linked to cell migration.
[ILLUSTRATIONS OMITTED]
Results of this study suggest that chlorpyrifos exposure, mediated through its effects on nAChRs and PKC, leads to a change in cell phenotype, since there are no pigmented cells in a normal embryo or larva of this sea urchin species, rather than causing a primary disruption of cell proliferation or migration. This is followed by generalized dysmorphology characterized by subsequent cell extrusion and the formation of extralarval caps containing pigmented cells. Since the extralarval caps can exceed 90% of the total number of larval cells, it appears that cells from different parts of the embryo or larva undergo the transformation. This suggests that the developmental targets of chlorpyrifos, including nAChRs and PKC, and potentially the monoamines and their signaling cascades (9,21,22), represent primary sites for regulation of cell phenotype. As well, our results address the time frame in which these processes are likely to control cell fate. In the sea urchin, this corresponds to the mid-blastula stage, the point at which the maternal genome is turned off and the embryonic (zygotic) genome is turned on (43-46,51). That this phenomenon is connected to compartmentalized augmentation of cholinergic signaling implies that the sea urchin embryo may ultimately provide us with the tools to characterize the molecular events underlying the neuroteratogenesis of a wide variety of drugs and environmental agents that converge on cholinergic signals or their downstream signaling cascades.
There are, of course, limitations to the use of the sea urchin as a potential model for screening and mechanistic studies of pesticide effects on mammalian CNS development. The sea urchin, like culture-based models, cannot elucidate the importance of maternal--fetal pharmacokinetics and metabolism in determining the concentration of neuroteratogens that reach the fetus. Accordingly, the thresholds necessary to elicit effects in sea urchins cannot provide an absolute guide to the appropriate calculation of exposure limits for regulatory purposes. On the other hand, the sensitivity of sea urchin embryos seen here is quite comparable to that with in vitro mammalian models, such as whole embryos, neuronal cell lines, or CNS cultures (11,12,14-19,31-34). Nevertheless, our results point to potential utility of this or similar invertebrate model systems to screen compounds for potential neuroteratogenic activity in a comparative manner [as done here for chlorpyrifos, chlorpyrifos oxon, and dieldrin, and in a preliminary study for diazinon (66)], and to help identify heretofore unsuspected cellular targets underlying neurobehavioral teratogenesis.
REFERENCES AND NOTES
(1.) Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Bodbold JH, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect 107(suppl 3):431-437 (1999).
(2.) Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health 2:161-181 (1999).
(3.) Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect 10(suppl 1):71-80 (1999).
(4.) Fenske RA, Black KG, Elkner KP, Lee C, Methner MM, Soto R. Potential exposure and health risks of infants following indoor residential pesticide applications. Am J Public Health 80:689-693 (1990).
(5.) Gurunathan S, Robson M, Freeman N, Buckley B, Roy A, Meyer R, Bukowski J, Lioy PJ. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environ Health Perspect 106:9-16 (1998).
(6.) Davis DL, Ahmed AK. Exposures from indoor spraying of chlorpyrifos pose greater health risks to children than currently estimated. Environ Health Perspect 106:299-301 (1998).
(7.) U.S. Environmental Protection Agency. Administrator's Announcement. Available: http://www.epa.gov/pesticides/announcement6800.htm [cited 11 October 2000].
(8.) Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol 134:53-62 (1995).
(9.) Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol 145:158-174 (1997).
(10.) Dam K, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: delayed targeting of DNA synthesis after repeated administration. Der Brain Res 108:39-45 (1998).
(11.) Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol 151:182-191 (1998).
(12.) Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: affects on nuclear transcription factor involved in cell replication and differentiation. Brain Res 857:87-98 (2000).
(13.) Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev Brain Res 121:179-187 (2000).
(14.) Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res 121:189-195 (2000).
(15.) Monnet-Tschudi F, Zurich MG, Schilter B, Costa LG, Honegger P. Maturation-dependent effects of chlorpyrifos and parathion and their oxygen analogs on acetylcholinesterase and neuronal and glial markers in aggregating brain cell cultures. Toxicol Appl Pharmacol 165:175-183 (2000).
(16.) Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol 160:217-230 (1999).
(17.) Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104:129-140 (1995).
(18.) Bagchi D, Bhattacharya B, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology 112:57-68 (1996).
(19.) Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Chlorpyrifos elicits mitotic abnormalities and apoptosis in neuroepithelium of cultured rat embryos. Teratology 58:62-68 (1998).
(20.) Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull 43:179-189 (1997).
(21.) Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev Brain Res 116:9-20 (1999).
(22.) Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos releases norepinephrine from adult and neonatal rat brain synaptosomes. Dev Brain Res 118:120-133 (1999).
(23.) Liu J, Pope CN. Effects of chlorpyrifos on high-affinity choline uptake and [[sup.3]H]hemicholinium-3 binding in rat brain. Fundam Appl Toxicol 34:84-90 (1996).
(24.) Chanda SM, Pope CN. Neurochemical and neurobehavioral effects of repeated gestational exposure to chlorpyrifos in maternal and developing rats. Pharmacol Biochem Behav 53:771-776 (1996).
(25.) Liu J, Pope CN. Comparative presynaptic neurochemical changes in rat striatum following exposure to chlorpyrifos or parathion. J Toxicol Environ Health 53:531-544 (1998).
(26.) Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther 269:329-335 (1994).
(27.) Huff RA, Abou-Donia MB. In vitro effect of chlorpyrifos oxon on muscarinic receptors and adenylate cyclase. Neurotoxicology 16:281-290 (1995).
(28.) Ward TR, Mundy WR. Organophosphorus compounds preferentially affect second messenger systems coupled to M2/M4 receptors in rat frontal cortex. Brain Res Bull 39:49-55 (1996).
(29.) Katz EJ, Cortes VI, Eldefrawi ME, Eldefrawi AT. Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol 146:227-236 (1997).
(30.) Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res 769:211-218 (1997).
(31.) Ehrich M, Correll L, Veronesi B. Acetylcholinesterase and neuropathy target esterase inhibitions in neuroblastoma cells to distinguish organophosphorus compounds causing acute and delayed neurotoxicity. Fundam Appl Toxicol 38:55-63 (1997).
(32.) Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett 98:139-146 (1998).
(33.) Cao CJ, Mioduszewski RJ, Menking DE, Valdes JJ, Katz EJ, Eldefrawi ME, Eldefrawi AT. Cytotoxicity of organophosphate anticholinesterases. In Vitro Cell Dev Biol 35:493-500 (1999).
(34.) Cosenza ME, Bidanet J. Effects of chlorpyrifos on neuronal development in rat embryo midbrain micromass cultures. Vet Human Toxicol 37:118-121 (1995).
(35.) Chaudhuri J, Chakraborti TK, Chanda S, Pope CN. Differential modulation of organophosphate-sensitive muscarinic receptor subtypes in rat brain by parathion and chlorpyrifos. J Biochem Toxicol 8:207-216 (1993).
(36.) Auman JT, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: implications for neurotoxicity. Dev Brain Res 121:19-27 (2000).
(37.) May M. Disturbing behavior: neurotoxic effects in children. Environ Health Perspect 108:A262-A267 (2000).
(38.) Padilla S, Buzzard J, Moser VC. Comparison of the role of esterases in the differential age-related sensitivity to chlorpyrifos and methamidophos. Neurotoxicology 21:49-56 (2000).
(39.) Barone S, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology 21:15-36 (2000).
(40.) National Research Council. Pesticides in the Diets of Infants and Children. Washington DC:National Academy Press, 1993.
(41.) Gustafson T, Toneby M. On the role of serotonin and acetylcholine in sea urchin morphogenesis. Exp Cell Res 62:102-117 (1970).
(42.) Buznikov GA, Kost AN, Kucherova NF, Mndzhoyan AL, Suvorov NN, Berdysheva LV. The role of neurohumours in early embryogenesis. 3. Pharmacological analysis of the role of neurohumours in cleavage divisions. Embryol Exp Morphol 23:549-569 (1970).
(43.) Buznikov GA. Sea urchin embryos as a test system to detect embryotoxicity of chemical compounds. Biol Int 8:5-8 (1983).
(44.) Buznikov GA. Neurotransmitters in Embryogenesis. Chur, Switzerland:Harwood Academic Press, 1990.
(45.) Buznikov GA, Shmukler YB, Lauder JM. From oocyte to neuron: do neurotransmitters function in the same way throughout development? Cell Mol Neurobiol 16:532-559 (1996).
(46.) Buznikov GA, Jokanovic M, Kovacevic N, Rakic L. Sea urchin embryos and larvae as biosensors for screening and detailed study of pharmacologically active substances. Arch Toxicol Kinet Xenobiotica Metab 5:393-400 (1997).
(47.) Buznikov GA, Rakic L Cholinoreceptors of early (pre-nervous) sea urchin embryos. Neurosci Behav Physiol 30:53-62 (2000).
(48.) Shmukier YB, Buznikov GA. Functional coupling of neurotransmitters with second messengers during cleavage divisions: facts and hypotheses. Perspect Dev Neurobiol 5:469-483 (1998).
(49.) Buznikov GA. Low Molecular Weight Regulators of Early Embryonic Development. Moscow:Nauka, 1967.
(50.) Hagstrom BE, Lonning S. The sea urchin egg as a testing object in toxicology. Acta Pharmacol Toxicol 32:1-39 (1973).
(51.) Buznikov GA, Podmarev VI. The sea urchins Strongylocentrotus droebachiensis, S. nudus and S. intermedius. In: Animal Species for Developmental Studies, Vol 1: Invertebrates (TA Dettlaff, Vassetzky SG, eds). New York-London:Consultants Bureau, 1990;251-283.
(52.) Liu JP, Brannen KC, Grayson DR, Morrow AL, Devaud LL, Lauder JM. Prenatal exposure to the pesticide dieldrin or the GABA(A) receptor antagonist bicuculline differentially alters expression of GABA(A) receptor subunit mRNAs in fetal rat brainstem. Dev Neurosci 20:83-92 (1998).
(53.) Brannen KC, Devaud LL, Liu JP, Lauder JM. Prenatal exposure to neurotoxicants dieldrin or lindane alters tertbutylbicyclophosphorothionate binding to GABA(A) receptors in fetal rat brainstem. Dev Neurosci 20:34-41 (1998).
(54.) Bezuglov VV, Manevich Y, Archakov AV, Bobrov MY, Kuklev DV, Petrukhina GN, Makarov VA, Buznikov GA. Artificially functionalized polyenoic fatty acids as new lipid bioregulators. Russ J Bioorg Chem 23:190-198 (1997).
(55.) Bezuglov VV, Zinchenko GN, Nikitina LA, Buznikov GA. Arachidonoylcholine and O-arachidonoyldimethylaminoethanol as new cholinergic substances. Russ J Bioorg Chem (in press).
(56.) Buznikov GA, Bezuglov VV. 5-Hydroxytryptamides and 3-hydroxytyramides of polyenoic fatty acids as a tool for studying the pre-nervous biogenic monoamines functions. Russ Physiol J 86:1093-1108 (2000).
(57.) Hunter DL, Lassiter TL, Padilla S. Gestational exposure to chlorpyrifos: comparative distribution of trichloropyridinol in the fetus and dam. Toxicol Appl Pharmacol 158:16-23 (1999).
(58.) Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther 287:1135-1144 (1998).
(59.) Slotkin TA, Orband-Miller L, Queen KL. Development of [[sup.3]H]nicotine binding sites in brain regions of rats exposed to nicotine prenatally via maternal injections or infusions. J Pharmacol Exp Ther 242:232-237 (1987).
(60.) Falugi C. Localization and possible role of molecules associated with the cholinergic system during "non-nervous" developmental events. Eur J Histochem 37:287-294 (1993).
(81.) Ivonnet PI, Chambers EL. Nicotinic acetylcholine receptors of the neuronal type occur in plasma membrane of sea urchin eggs. Zygote 5:277-287 (1997).
(62.) Narahashi T, Ginsburg KS, Nagata K, Song JH, Tatebayashi H. Ion channels as targets for insecticides. Neurotoxicology 19:581-590 (1998).
(63.) Ward KW, Rogers EH, Hunter ES. Dysmorphogenic effects of a specific protein kinase C inhibitor during neurulation. Reprod Toxicol 12:25-34 (1998).
(64.) Steingart RA, Silverman WF, Barron S, Slotkin TA, Awad Y, Yanai J. Neural grafting reverses prenatal drug-induced alterations in hippocampal PKC and related behavioral deficits. Dev Brain Res 125:9-19 (2001).
(65.) Steingart RA, Barg J, Maslaton J, Nesher M, Yanai J. Pre- and postsynaptic alterations in the septohippocampal cholinergic innervations after prenatal exposure to drugs. Brain Res Bull 46:203-209 (1998).
(66.) Morale A, Coniglio L, Angelini C, Cimoli G, Bolla A, Alleteo D, Russo P, Falugi C. Biological effects of a neurotoxic pesticide at low concentrations on sea urchin early development: a teratogenic assay. Chemosphere 37:3001-3010 (1998).
Address correspondence to T. Slotkin, Department of Pharmacology and Cancer Biology, Box 3813 DUMC, Duke University Medical Center, Durham, NC 27710-3813 USA. Telephone: (919) 681 8015. Fax (919) 684 8197. E-mail: t.slotkin@duke.edu
This research was supported by U.S. Public Health Service ES10356, ES10387, ES10159 and by the Russian Foundation for Basic Research (project #99-04-48514).
We thank D. McClay, Duke University, for helpful comments and D.L. Hunter, U.S. Environmental Protection Agency, for assistance with the chlorpyrifos oxon assays.
This paper has been reviewed by the Health Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. Environmental Protection Agency, and mention of trade names of commercial products does not constitute endorsement or recommendation for use.
Received 17 October 2000; accepted 4 January 2001.
Gennady A. Buznikov,(1) Lyudmila A. Nikitina,(1) Vladimir V. Bezuglov,(2) Jean M. Lauder,(3) Stephanie Padilla,(4) and Theodore A. Slotkin(5)
(1) N.K. Koltzov Institute of Developmental Biology, Russian Academy of Sciences, Moscow, Russia; (2) Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia; (3) Department of Cell Biology and Anatomy, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA; (4) National Health and Environmental Effects Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina, USA; (5)Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, North Carolina, USA
COPYRIGHT 2001 National Institute of Environmental Health Sciences
COPYRIGHT 2004 Gale Group