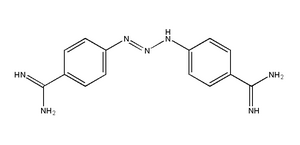
ABSTRACT
WESONGAH, J.O., MURILLA, G.A., KIBUGU, J.K. & JONES, T.W. 2004. Evaluation of isometamidium levels in the serum of sheep and goats after prophylactic treatment against trypanosomosis. Onderstepoort Journal of Veterinary Research, 71:175-179
Isometamidium chloride has been used for the control of trypanosomosis in animals for over 36 years, but recently there have been reports of prophylaxis failure under natural conditions. In this study, use of the drug for prophylactic purpose against trypanosomosis in small ruminants was investigated. Forty-two sheep and 44 goats were divided into four treatment groups. Groups 1 and 2 were treated with isometamidium chloride (Samorin®, Rhone Merieux, Lyon, France) at 3-month intervals while groups 3 and 4 were used as controls. All the animals were exposed to natural tsetse challenge and monitored for serum isometamidium levels and anti-trypanosome antibodies.
Seven days after drug administration, isometamidium levels were significantly higher in goats 13.7 ± 0.07 ng/ml than in sheep 6.2 ± 0.06 ng/ml. However, the elimination half-life in the sheep was 14.2 ± 0.92 days and was significantly higher (P > 0.05) than that of the goats 12 ± 0.5 days. This study established that isometamidium metabolism differs between sheep and goats and this difference may have important implications in high tsetse challenge areas.
Keywords: ELISA, goats, isometamidium chloride, sheep, trypanosomosis
INTRODUCTION
Trypanosomosis in animals is a major constraint to livestock production in large areas of sub-Saharan Africa (Spath 2000). Only the trypanotolerant breeds can survive, reproduce and remain productive in tsetse-infested areas with a minimum requirement of trypanocidal drug treatment (Murray, Morrison & Whitelaw 1982). lsometamidium chloride is the main drug that is used for prophylaxis against trypanosome infections in livestock in Africa.
A number of studies have been carried out to investigate the use of isometamidium chloride in sheep and goats under natural tsetse challenge (Masiga, Okech, lrungu, Ouma, Wekesa, Ouma, Guya & Ndungu 2001; Okech, Masinde, Stevenson & Ndungu 1997; Griffin & Allonby 1979a). The studies showed that sheep and goats could be protected against trypanosomosis by isometamidium chloride for periods ranging from 6 to 16 weeks. In the present study isometamidium-enzyme-linked immunosorbent assay (Isometamidium-ELISA) was used to determine isometamidium concentrations in the serum of sheep and goats.
Previously, Braide & Eghianruwa (1980) attempted to evaluate isometamidium residues in goat tissues using spectrophotometric methods. They were, however, unable to follow the drug for long periods due to limitations of the methods used. In the present study more data on serum isometamidium concentrations was collected using the isometamidium-ELISA described by Eisler, Elliott & Holmes (1996) which has a detection limit of 0.1 ng/m£.
MATERIALS AND METHODS
Study area
The study was carried out at the National Range Research Centre (NRRC) of the Kenya Agricultural Research Institute (KARI) in Kiboko area of Makueni district, Eastern Province of Kenya. The study area comprises grass savannah and Acacia-Commiphora bushland typical of ecological Zone V, as classified by Pratt, Greenway & Gwynne (1966), with a low erratic annual rainfall. The tsetse population in Kiboko area consists principally of Glossina pallidipes, Glossina longipennis and Glossina brevipalpis. Glossina pallidipes is the most common, followed by G. brevipalpis (Kiragu 1997; Griffin & Allonby 1979b). However, recently Kiboko area has been reported to have a very low tsetse challenge (Murilla, Mdachi, Wesongah & Karimi 2003).
The NRRC management has instituted a programme in which sheep and goats are protected against trypanosomosis by prophylactic treatment with isometamidium chloride at a dose of 1 mg/kg body mass every 3 months.
Experimental animals
Experimental animals, comprising two different breeds of sheep (Red Maasai and Black Head Somali) weighing 18-36 kg and goats (Small East Africa and GaIIa) weighing 12.5-33 kg, were used (Table 1). Twenty-eight sheep were randomly selected from a population of 38 sheep at the KARI-Kiboko NRRC field station. However, all 28 goats available on the ranch were used. Fourteen sheep and 16 goats were purchased from farmers in the vicinity of the ranch and used as untreated controls. The experimental animals were identified by ear tags and left to graze under natural conditions. The animals were dewormed with 1.5 % levamisole hydrochloride and 3 % w/v oxyclozanide (Levafas, Norbrook Laboratories Ltd) at 1 ml/2 kg body mass once every 3 months, and dipped monthly using an acaricide (Stelladoneâ, Ciba-Geigy, Switzerland) to control ticks and tick-borne diseases. Prior to the start of the experiments all the animals were screened for anti-trypanosome antibodies against Trypanosoma congolense, Trypanosoma brucei and Trypanosoma vivax using an antibody trapping ELISA as described by Masake, Moloo, Nantulya, Minja, Makau & Njuguna (1995). As T. congolense antigen is known to cross-react with antibodies produced against T. wVaxand T. brucei(Luckins 1977), this test could be used to screen animals for any previous exposure to any of the three trypanosome species. All the animals were weighed monthly using a mechanical weighing balance.
Drug administration
Isometamidium chloride (Samorin®, Rhone Merieux, Lyon, France) was administered at 3-monthly intervals. The drug was prepared immediately before use as a 2% w/v solution in distilled water and was given as a single intramuscular injection into the neck muscles at a rate of 1 mg/kg body mass. Controls were not treated.
Experimental design
The animals were divided into four treatment groups as shown in Table 1. All the animals on the ranch, including those that were not used for the experiment, were grazed together to ensure they were exposed to similar tsetse challenge.
Serum collection
Blood samples were collected 2 days before treatment to determine anti-trypanosome antibody levels and for use in the preparation of isometamidium standards as described by Eisler et al. (1996). Following the treatment, blood samples for serum preparation were collected weekly to determine drug concentrations as described by Eisler et al. (1996) and for determination of the presence of anti-trypanosome antibodies using the Antibody-ELISA as described by Masake et al. (1995).
Drug analysis
Isometamidium-enzyme-linked immunosorbent assay (ELISA)
Sera prepared from sheep and goats after treatment were tested using the isometamidium-ELISA (Eisler et al. 1996) without any modification.
Cross-reactivity (specificity)
The cross-reactivity of isometamidium reagents with other trypanocides, including homidium bromide and diminazene aceturate, was determined by analyzing negative control serum spiked with these drugs at concentrations varying from 1 ng/ml to 1 mg/mA Cross-reactivity was calculated as the ratio of the concentration of isometamidium and cross-reacting drug that gave optical densities (B) in the ELISA that were half that of serum without drugs (Bo). Specific detection of isometamidium was obtained and no significant cross-reactivity with homidium bromide or diminazene aceturate was observed. Neither aqueous solutions of the drugs nor sera from animals treated with diminazene aceturate or homidium yielded a positive signal in the ELISA.
Limit of detection
The limit of detection was established by measuring the optical densities (OD) of 12 negative control serum samples collected from sheep or goats in a trypanosomosis-free area. The value for limit of detection was taken as the concentration equivalent to the mean OD minus three standard deviations. Limit of detection was also used as limit of quantification in all assays.
Quality control
In all assays, quality control standards were included in each microtitre plate as markers of unexpected assay variations and to monitor assay performance. During the analysis the quality control standards prepared from serum collected 2 days following isometamidium administration and a negative control serum sample were included on every plate.
Background
Background effects of the assay were eliminated by incubating the samples overnight to allow for maximum binding of the entire drug in the sample to the antibody coated on the plate. Any unbound material was washed off before addition of the drug conjugate.
Detection of anti-trypanosome antibodies
The antibody detection ELISA technique described by Masake et al. (1995) was used to assess the presence of anti-trypanosome antibodies in all the sheep and goats over the experimental period of over 80 days following each treatment.
A checker board titration was used to determine the optimum conjugate dilutions using known negative and positive control serum samples. A cut-off point of OD values was established to be 0.35 (n = 50) and 0.75 (n = 50) for sheep and goats respectively, using frequency distribution curves.
The negative and positive control sera were obtained from all the experimental sheep and goats before treatment. The pre-treatment sera of both sheep and goats used in the study gave a mean OD of 0.15, and hence OD values > 0.20 were regarded as ELISA positive.
Data analysis
Data collected included serum isometamidium concentrations and antibody levels. Mean ±SD values were calculated for all data sets. The values were compared within and between various treatment groups (at 95 % confidence interval) using the Welch's corrected t test (lnstat biostatistics software).
An estimate of the rate of decline of isometamidium over this period (7-105 days) was obtained by performing linear regression (log mean concentrations versus time after treatment) for individual sheep and goats.
RESULTS
Anti-trypanosome antibodies
All the control sheep and goats were negative for anti-trypanosome antibodies while 10% and 14% of the isometamidium treated goats and sheep, respectively, were positive prior to the start of the experiments, indicating previous exposure to trypanosome infections.
Serum isometamidium concentration
Sheep
The decline of the mean serum isometamidium concentration over time is shown in Fig. 1. A mean serum isometamidium concentration of 6.2 ± 0.06 ng/ml was detected 7 days after treatment, which declined to 3.0 ± 1.5 ng/ml and 1.2 ± 0.03 ng/ml by 14 and 29 days respectively after treatment. A concentration of 0.4 ± 0.26 ng/m/ was still detectable in 21 % of the treated sheep up to 77 days after treatment and was undetectable in all the sheep at 105 days after treatment. An apparent drug elimination rate ranging from 8.8-29.8 days (mean = 14.2 ± 0.92 days) was estimated in sheep using linear regression (Fig. 1).
Goats
The decline in the mean serum isometamidium concentration over time is shown in Fig. 1. A mean concentration of 13.7 ± 0.07 ng/mt was detected 7 days after treatment. This drug concentration then declined to 9.5 ± 3.8 ng/ml and 4.3 ± 0.03 ng/mf at 14 and 29 days respectively after treatment. Mean serum isometamidium concentrations of 0.43 ± 0.37 ng/ml were still detectable in approximately 46% of the isometamidium-treated goats at 77 days after treatment. However, the drug was undetectable in all the goats at 105 days after treatment. An apparent drug elimination half-life of approximately 7.4-21 days (mean = 12 ± 0.5 days) was estimated in goats (Fig-1).
In comparison, the mean serum isometamidium concentrations were significantly higher (P
DISCUSSION
The current study has established that serum isometamidium levels of 0.4 ± 0.26 ng/ml and 0.43 ± 0.37 ng/ml could be detected in 21 % sheep and 46% of goats for periods up to 77 days respectively after prophylactic treatment. The drug concentrations were persistently higher in goats than in sheep over the observation period. Previously, serum isometamidium concentration in goats had not been described beyond 48 h (Kinabo & McKellar 1990; Braide & Eghianurwa 1980) and there is no information on isometamidium persistence in sheep. The availability of ELISA for isometamidium has, therefore, made it possible to establish the drug profiles in sheep and goats.
The results from studies carried out by Eisler, Arowolo, Gault, Moloo, Holmes, Peregrine (1994), using the isometamidium-ELISA, showed that isometamidium was detectable in serum of treated cattle up to 3 months after treatment. In the present study, serum isometamidium concentrations in both sheep and goats were 0.5 ± 0.08 ng/ml and 0.6 ± 0.02 ng/mf at 49 and 63 days respectively. These levels exceed the protective levels reported in cattle after prophylactic treatment (Eisler et al. 1994). However, even in the absence of detectable isometamidium concentrations, cattle can still be protected by the drug (Geerts, Kageruka, De Deken, Brandt, Kazadi, Diarra, Eisler, Lemmouchi, Schacht & Holmes 1997).
The serum isometamidium concentration versus time profiles established in the present study differed markedly from those reported previously. For instance, Braide & Eghianruwa (1980) reported mean serum isometamidium concentration of 2.17 µg/ml at 24 h after intramuscular administration of isometamidium chloride in goats at a dosage rate of 0.5 mg/kg body mass. Subsequent serum samples taken at 7 days, 2 weeks and 3 weeks after a single dose injection did not contain any detectable amounts of the drug, presumably because of the low sensitivity of the analytical method used. In the present study serum isometamidium levels were higher in goats than in sheep throughout the experimental period. However, the elimination half-life of the drug in sheep (14.2 ± 0.92 days) was higher as compared to goats (mean = 12 ± 0.5 days) suggesting that isometamidium persisted longer in sheep than in goats under the conditions of the study. Implications of these observations in chemoprophylaxis need to be investigated further in an area of high tsetse challenge.
ACKNOWLEDGEMENTS
We thank the Department for International Development (DFID) and the Kenya Government through KETRI for funding this project, the Residue Analysis staff for their assistance with this work and Dr S.K. Karimi (KARI) for his professional assistance. We also thank Dr Mark Eisler for the ELISA software, isometamidium conjugate and antibody. We are grateful to Drs J.M. Ndungu, O.K. Masiga and S. Nyamwaro for constructive criticism of the paper. This paper is published with the kind permission of the Director, KETRI.
REFERENCES
BRAlDE, V.B. & EGHIANRUWA, K.I. 1980. lsometamidium residues in goat tissues after parenteral administration. Research in Veterinary Science, 29:111-113.
EISLER, M.C., AROWOLO, R.O.A., GAULT, E.A., MOLOO, S.K., HOLMES, P.H. & PEREGRINE, A.S. 1994. Isometamidium concentrations in the sera of Boran cattle: Correlation with prophylaxis against tsetse-transmitted Trypanosoma congolense. Acta Tropica, 56:39-50.
EISLER, M.C., ELLIOTT, C.T. & HOLMES, P.M. 1996. A simple competitive enzyme-linked immunoassay for the detection of the trypanocidal drug isometamidium. Therapeutics Drug Monitoring, 18:73-79.
GEERTS, S., KAGERUKA, P., DE DEKEN, R., BRANDT, J.R., KAZADI, J.M., DIARRA, B., EISLER, M.C., LEMMOUCHI, Y., SCHACHT, E. & HOLMES, P.H. 1997. Prophylactic effects of isometamidium- and ethidium-sustained release devices against Trypanosoma congolense in cattle. Acta Tropica, 65:23-31.
GRIFFIN, L. & ALLONBY, E.W. 1979a. The economic effects of trypanosomiasis in sheep and goats at a range station in Kenya. Tropical Animal Health and Product/on, 11:127-132.
GRIFFIN, L. & ALLONBY, E.W. 1979b. Studies on the epidemiology of trypanosomiasis in sheep and goats in Kenya. Tropical Animal Health and Production, 11:133-142.
KINABO, L.D.B. & MCKELLAR, Q.A. 1990. Isometamidium in goats: Disposition kinetics, mammary excretion and tissue residues. British Veterinary Journal, 146:405
KIRAGU, J.M. 1997. Ecology and behaviour of the tsetse fly Glossina brevipalpis Newstead and its possible role as a vector of livestock trypanosomiasis in Kibwezi, Kenya. Ph.D thesis, University of Bristol.
LUCKINS, A.G. 1977. Detection of antibodies in trypanosome-infected cattle by means of microplate enzyme-linked immunosorbent assay (ELISA). Tropical Animal Health and Production, 9:53-62.
MASAKE, R.A., MOLOO, S.K., NANTULYA, V.M., MINJA, S.H., MAKAU, J.M. & NJUGUNA, JT. 1995. Comparative sensitivity of antigen-detection enzyme immunosorbent assay and microhaematocrit centrifugation technique in the diagnosis of Trypanosoma brucei infections in cattle. Veterinary Parasitology, 56:37-46.
MASIGA, O.K., OKECH, G., IRUNGU, P., OUMA, J.O., WEKESA, S., OUMA, B., GUYA, S.O. & NDUNGU, J.M. 2001. Growth and mortality in sheep and goats under high tsetse challenge in Kenya. Tropical Animal Health and Production, 34: 489-501.
MURILLA, G.A., MDACHI, R.E., WESONGAH, J.O. & KARIMI, S.K. 2003. An investigation into trypanocidal drug resistance in cattle on the KARI, National Range Research Station, Kiboko, Makueni district, Eastern province. ARF End of Project Conference, 2-4 December 2003.
MURRAY, M., MORRISON, W.I. & WHITELAW, D.D. 1982. Host susceptibility to African Trypanosomosis: trypanotolerance. Advanced Parasitology, 21:1-68.
OKECH, G., MASINDE, A., STEVENSON, P. & NDUNGU, J.M. 1997. The role of isometamidium chloride in chemoprophylaxis against trypanosomiasis of small ruminants in Kenya. Proceedings of the 24th meeting of the International Scientific Council for Trypanosomiasis Research and Control (ISCTRC), Maputo, Mozambique.
PRATT, D.J., GREENWAY, PJ. & GWYNNE, M.D. 1966. A classification of East African rangeland with an appendix on terminology. Journal of Applied Ecology, 3:369-382
SPATH, J. 2000. Feeding patterns of the three sympatric tsetse species (Glossina spp.) (Diptera, Glossinidae) in the preforest zone of Cote d'Ivoire. Acta Tropica, 75:109-118.
J.O. WESONGAH1*, G.A. MURILLA1, J.K. KIBUGU1 and T.W. JONES2
* Author to whom correspondence is to be directed. E-mail: jwesongah @ hotmail.com
1 Kenya Trypanosomiasis Research Institute, P.O. Box 362, Kikuyu, Kenya
2 The University of Edinburgh, Centre for Tropical Veterinary Medicine, Edinburgh, EH25 9RG Scotland (UK)
Accepted for publication 27 January 2004-Editor
Copyright Onderstepoort Veterinary Institute Sep 2004
Provided by ProQuest Information and Learning Company. All rights Reserved