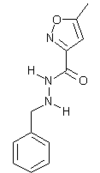
Marplan
Isocarboxazid is a nonselective hydrazine-derived monoamine oxidase inhibitor used in treatment resistant depression. more...
Uses
Approved
In the United States, isocarboxazid is approved for the treatment of depression.
Off-Label/Unapproved/Investigational
A randomized controlled trial published in December of 1988 found that isocarboxazid significantly reduced bingeing and purging in bulimia nervosa, regardless of the presence or absence of depression or personality disorder.
Brand Names
United States
MARPLAN® by Hoffmann-La Roche, starting in 1959 and discontinuing in 1994. After much patient and physician outcry, they resumed manufacturing just enough Marplan® to distribute on an as-needed basis. In October of 1998, Hoffmann-La Roche and Oxford Pharmaceutical Services Inc. announced that Oxford, "is acquiring ownership of the NDA for the antidepressant product Marplan® (isocarboxazid) from Roche."
The maximum daily dose of isocarboxazid is 60mg.
Read more at Wikipedia.org