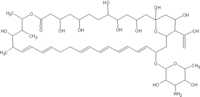
Mykacet
Nystatin is a polyene antimycotic drug. Nystatin is considered a "clean" drug as it has no proven side effects. Candida spp. are sensitive to nystatin. more...
Uses
Cutaneous, vaginal, mucosal and esophageal Candida infections can be treated with nystatin. Cryptococcus is also sensitive to nystatin. In the UK its license for treating neonatal oral thrush is restricted to those over the age of one month (miconazole is an appropriate alternative for younger babies).
Nystatin is often used as prophylaxis in patients who are at risk for fungal infections, such as AIDS patients with a low CD4+ count and patients receiving chemotherapy.
It is prescribed in units, with doses varying from 100,000 (for oral infections) to 1 million (for intestinal ones). As it is not absorbed from the gut, it is safe for oral use and does not have probelms of drug interactions.
Method of action
Like amphotericin B and natamycin, nystatin binds to ergosterol, the main component of the fungal cell membrane. When present in sufficient concentrations, it forms a pore in the membrane that leads to K+ leakage and death of the fungus. As mammals do not have ergosterol-based cell membranes, the drug only affects fungi.
Origin
Like many other antimycotics and antibiotics, nystatin is itself a fungal product. It was isolated from Streptomyces noursei in 1950 by Elizabeth Lee Haxen and Rachel Fuller Brown, who were doing research for the Division of Laboratories and Research of the New York State Department of Health. The soil sample where they discovered nystatin, was from the garden of Hazen's friends called Nourses, therefore the strain was called noursei. Hazen and Brown named nystatin after the New York State Public Health Department.
Brand names
- Nystan® (oral tablets, topical ointment, and pessaries, Bristol-Myers Squibb)
- Infestat®
- Nystamont®
- Nystop® (topical powder, Paddock)
- Nystex®
- Mykinac®
- Nysert® (vaginal suppositories, Procter & Gamble)
- Nystaform® (topical ointment, combined with iodochlorhydroxyquine and hydrocortisone; Bayer)
- Nilstat® (vaginal tablet, Lederle)
- Korostatin® (vaginal tablets, Holland Rantos)
- Mycostatin® (vaginal tablets, Bristol-Myers Squibb)
- Mycolog-II® (topical ointment, combined with triamcinolone; Apothecon)
- Mytrex® (topical ointment, combined with triamcinolone)
- Mykacet® (topical ointment, combined with triamcinolone)
- Myco-Triacet II® (topical ointment, combined with triamcinolone)
Read more at Wikipedia.org