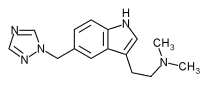
Merck received Food and Drug Administration approval to market Maxalt(r) (rizatriptan benozate) for acute treatment of migraines, with or without aura, in adults. It is available as a conventional tablet and a convenient tablet that disintegrates on the tongue within seconds.
The orally disintegrating version is named Maxalt-MLT(tm). It does not enhance absorption nor induce a quicker onset of action It merely offers convenience by enabling patients to take their migraine medication without water. Labeling indicates that peak plasma concentration (Cmax) for Maxalt tablets was reached in 1 to 1.5 hours, whereas Maxalt-MLT peaked slower, with Cmax attained at 1.6 to 2.5 hours. Both tablet forms are available in strengths of 5 mg and 10 mg.
Maxalt is indicated for migraine pain, as well as other migraine symptoms, such as nausea, photophobia and phonophobia. Side effects, which included dizziness, drowsiness and fatigue, were reported to be mostly transient and dose-related. Contraindications include uncontrolled high blood pressure, heat disease or history of heart disease and concomitant use of monoamine oxidase inhibitors. Maxalt should not be taken within 24 hours of treatment with ergotamine-type drugs or within 14 days of taking an MAOI.
COPYRIGHT 1998 Reproduced with permission of the copyright holder. Further reproduction or distribution is prohibited without permission.
COPYRIGHT 2004 Gale Group