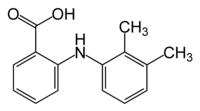
The quality of a solid pharmaceutical preparation is influenced by primary micromeritic characteristics such as the shape and size of drug crystals, especially when large amounts of poorly soluble drugs are formulated. To improve the dissolution rate of poorly soluble drugs, fine crystals ale preferred over large crystals because they provide a greater surface area. However, micronization can change drugpowder properties such as wettability, compressibility, packability, and flow and thus prevent efficient powder packaging. For that reason, it is more beneficial to transform the microcrystalline drug itself into an agglomerated form using a crystallization process. The resulting spherically agglomerated crystals then can be prepared in tablet form or compounded directly into a pharmaceutical system without further processing such as granulation. This approach also eliminates steps in the tableting process by making it possible to use direct tableting. Direct tableting, in which powders are simply mixed and compressed, generally is preferred because it saves time and reduces cost in comparison with the granulation technique.
Flowability is an important characteristic for materials to be compressed as tablets. Spheres are the ideal physical shape for this purpose because they reduce the amount of contact with the walls of the machine parts. The spherical crystallization technique also involves the use of a bridging liquid that improves compressibility by acting as granulating fluid. Thus spherical crystallization is a method that helps achieve good flowability and compressibility.
Spherical crystallization can be achieved by various methods such as simple spherical crystallization, emulsion solvent diffusion, ammonia diffusion, and neutralization (1,2). In this study the ammonia diffusion method was used, in which acetone, used as a solvent, enters a droplet of ammonia water and ammonia is liberated from the acetone-ammonia water-chloroform system (3). The three stages of the crystallization process are shown in Figure 1. First, the drug dissolved in ammonia water is precipitated while the droplets collect the crystals (Figure 1a). Simultaneously, ammonia in the agglomerate diffuses to the outer organic solvent (Figure 1b). Its ability to act as a bridging liquid weakens and subsequently spherical agglomerates are formed (Figure 1c). The drug mefenamic acid (MA) was selected for this study because it is poorly compressible, requires a high dose, and is poorly soluble in water. The aim of this study was to prepare spherical crystals of MA to obtain a directly compressible material for the preparation of tablets with improved bioavailability.
[FIGURE 1 OMITTED]
Materials and methods
The drug mefenamic acid was a gift from Blue Cross Laboratories (Goa, India). Its purity was confirmed by testing its melting point and by examining it using the IR spectrum. All the solvents were of pure laboratory grade and were purchased from CDH (Mumbai, India).
Preparation of the spherical crystals, In this study the ammonia diffusion method reported by Kawashima et al. was used for the spherical crystallization of MA (3). The drug was dissolved in a strong ammonia solution (25%) and mixed with 10 mL of acetone. Then chloroform was added with continuous stirring using a three-blade agitator in a cylindrical vessel. The agglomerated crystals were collected by filtration and washed with chloroform. Afterward, the crystals were dried at 50 [degrees]C and stored in a tightly closed container.
The effect of various parameters on the yield of spherical crystals was studied. These parameters included the amount of ammonia water used as a bridging liquid (10, 15, and 20 mL); the agitation speed (1000, 1500, and 2000 rpm); the agitation time (30, 60, and 90 min) and the concentration of ammonia in ammonia water (10, 15, 20, and 25%). The effects were tested by changing one parameter at a time while keeping the other parameters constant. The parameters that yielded the best formulation were used for further preparation of the spherical crystals.
Characterization of the spherical crystals. The physical properties of the spherical crystals formed were compared with those of MA powder. The purity of spherical crystallization was determined by IR spectrum analysis (Perkin Elmer IR spectrometer) and melting point determination (Toshniwall apparatus). The IR spectrum of the MA spherical crystals was recorded to monitor the potential formation of polymorphs and other changes in the structure of the compound resulting from the crystallization process. Solubility was determined quantitatively using distilled water and 0.1 N hydrochloric acid at room temperature (25 [degrees]C). The excess drug was added to several vials containing 10 mL of the solvent. The suspensions were shaken at 25 [degrees]C for 24 h. The samples were then centrifuged, aliquots of the supernatants were evaporated, and the residue was dissolved in a minimum quantity (1 mL) of 25% sulphuric acid and diluted with distilled water. The MA content in the medium was analyzed spectrophotometrically at 287 nm using a Shimadzu 1601 spectrophotometer.
The average particle size distributions of the MA powder and its spherical crystals were determined using an optical microscope. The shape and surface of agglomerates were determined using both optical and scanning electron microscopes. The surface areas of the MA powder and the MA spherical crystals were calculated using the formula [pi][d.sup.2], in which d represents the diameter of particles. The true densities of MA and its spherical crystals were determined with the use of a relative-density bottle (4). For the determination of bulk density, 3 g of MA powder or its spherical crystals were placed in a 25-mL graduated cylinder. The cylinder was dropped onto a hard-wood surface from a height of i in. at intervals of 2 s. The bulk densities were then obtained by dividing the weight of the samples by the final volume of the samples contained in the cylinders. Flowabilities were measured in terms of angle of repose using the fixed funnel method. Compressibility values were determined by compressing the MA powder and the MA spherical crystals separately using a single-punch machine. Wettability values were determined indirectly by measuring the densities and surface tensions of the saturated aqueous solutions of MA powder and its spherical crystals (5). The densities were determined using a relative-density bottle. The surface tension values were determined using a stalagmometer. The porosities of the drug powder and the spherical crystals were calculated from their bulk and true densities. The porosity of the tablets was calculated from apparent density of the tablets using the following equation:
Porosity ([epsilon]) - 1- (apparent density/true density)
The contact angles of the saturated aqueous solutions of MA and its spherical crystals were determined by measuring the height of a large drop when it was placed on a tablet surface. The contact angle was calculated using the following equation:
[MATHEMATICAL EXPRESSION NOT REPRODUCIBLE IN ASCII]
in which B = [rho]g/2[gamma]; [gamma] is the surface tension of the saturated solution of the sample in water, dyne/cm; p is the density of the saturated solution of drug in water, g/[cm.sup.3]; E is the porosity of the tablet; and h is the height of the liquid drop, cm.
Formulation and characterization of the tablets. Tablets of both the drug powder and its spherical agglomerates (SA) were prepared with and without excipients. The names assigned to the tablet formulations are listed in Table I. Tablet hardness was determined using a Monsanto hardness tester (6). The tensile strength (T) of the compact was calculated using the following equation:
T = 2F/[pi]Dt
in which D and t are the diameter and thickness of the compact, respectively, and F is the force fracturing the compact. The friability values of the tablets were determined using a Roche-type friabilator. It was rotated at 25 rpm for 4 min. Percent friability was calculated using the following equation:
Friability = ([[W.sub.0] - W]/[W.sub.0]) x 100
in which [W.sub.0] is the weight of the tablets at time zero before revolution, and W is the weight of the tablets after 100 revolutions.
The disintegration times of the tablet formulations were determined using a tablet disintegration test apparatus (Veego, Mumbai). After tablet strength and other characteristics of the formulations were considered, tablets MA-B and SA-B were selected for dissolution study. The dissolution test was conducted using USP XX dissolution apparatus l. The amount of dissolved drug was determined spectrophotometrically at 287 nm. The same procedure was repeated using water and 0.1 N HCl as the dissolution medium. The tablet formulations were subjected to physical stability tests under various stress conditions to study the effect of temperature and humidity. The tablets were examined for changes in tablet color.
The chemical stability of the tablet formulations also was studied for a period of 3 weeks at 4 [+ or -] 1 [degrees]C and 50 [+ or -] 1 [degrees]C in the dark and at room temperature (25 [degrees]C) in ordinary daylight conditions. The initial drug content of each sample was considered to be 100%. The subsequent amounts remaining in the tablets were determined and the percentages of residual drug were calculated. A graph was plotted showing log (percent residual drug) and time. The shelf life, or [t.sub.10%] (the time required for the degradation of 10% drug) for each formulation was calculated using the equation
[t.sub.10%] = 0.104/k
Results and discussion
The ternary solvent system (i.e., partially miscible solvents, ammonia water, acetone and chloroform) was used to produce spherical crystals of MA. A triangular phase diagram was prepared to determine the proportions of the solvents that would produce the highest yield of crystals. Ammonia water, which is also a good solvent for MA, was used as a bridging liquid. Acetone causes crystallization of MA as it diffuses out from the droplet when chloroform is added. The agglomeration mechanism is explained with the help of photomicrographs (see Figure 2). This mechanism is divided into three zones: a zero-growth zone, a fast growth zone, and constant-growth zone (Figures 2a-c). Aggregation began immediately following the addition of the bridging liquid, and the spherically agglomerated crystals gradually formed by the coalescence of dispersed crystals until the dispersing medium was saturated with the bridging liquid. The particle size of the primary crystals in the agglomerates increased until the dispersing medium was saturated with the bridging liquid introduced. After saturation, primary crystal growth ceased. Before saturation of the dispersing medium with the bridging liquid, the bridging liquid introduced into the system was immediately dispersed and wet the surface of the original crystals. On the surface of the crystals, an adsorbed bridging liquid solution layer was formed. At the same time, the bridging liquid diffused into the dispersing medium from its position as an adsorbed layer on the crystal surface and the cross-contact point between particles. These diffusion processes induced a solubility change in the bridging liquid, resulting in the recrystallization (reprecipitation) of the dissolved crystalline material, which occurred to a greater degree on coarse particle surfaces than it did on smoother surfaces.
[FIGURE 2 OMITTED]
The amount of bridging liquid is a critical parameter in the spherical crystallization technique. When 10 mL of ammonia water was used, no agglomeration occurred. This may be because only a very small amount of bridging liquid was available for solubilization, leading to incomplete wetting of the agglomerates. When more than 20 mL of ammonia water was used, large agglomerates formed. When 15 mL of ammonia water was used, uniform spherical crystals were produced. This finding indicates that agglomeration might result from the coalescence of agglomerates with the liberated bridging liquid, the rate of which depends on the frequency of the collisions of agglomerates. Therefore, the agglomerates grew in size in proportion to the agitation speed up to a certain limit. Uniform spherical crystals were produced at an agitation speed of 1500 rpm. With increasing agitation speed, the average diameter of agglomerates decreased, and their shape became increasingly irregular. At a high agitation speed, the thickness of the bridging liquid layer adsorbed on the surface may have decreased, leading to a reduction in the amount of crystals produced. Further, the diffusion of bridging liquid from the surface of the particle into the outer medium increased, thereby reducing the growth rate of the primary crystals. The agglomeration of primary crystals always occurred by recrystallization and coalescence, irrespective of the agglomeration conditions. Once the dispersing medium was saturated with the bridging liquid, the increase in primary crystal size and the decrease in primary crystal number ceased because the diffusion-controlled recrystallization and fusion stopped. The bridging liquid introduced into the dispersing medium after the saturation point was immiscible in the system, and only coalescence of agglomerates with the bridging liquid (liberated from the system) occurred, causing an increase in agglomerate size. When agitation speed was increased, the agglomeration rate increased because of the increased rate of collision and coalescence of particles. The optimum agitation time for the spherical crystals to remain suspended in the solvent mixture was 55-60 min at 25 [+ or -] 2 [degrees]C. With shorter time durations, the formation of spherical crystals was incomplete. When the agitation time was increased beyond this point, a breakdown of the agglomerates took place. Also, as temperature was increased, large agglomerates were formed. This was probably a result of the increased solubility of the drug at high temperatures. Therefore the solubility temperature is an important process parameter for spherical crystallization. When the concentration of ammonia in ammonia water was 25%, spherical crystals were formed, whereas when the ammonia concentration was less than 25%, spherical crystals were not produced.
The IR spectra of the spherical crystals showed that no change occurred in the chemical nature of the drug. The melting point of the spherical crystals (230 [degrees]C) also confirmed that the chemical structure had not changed and that no impurities were present (7). Thus the procedure used for the preparation of spherical crystals involved only physical interactions of particulate materials, rather than chemical interactions, and yielded pure product.
The simple photomicrographs and scanning electron microscopy (SEM) photographs (see Figure 3) show that uniform spherical crystals of
MA were formed. The particle-size study of MA powder and spherical crystals showed the formation of agglomerates. A study of the surface area followed the micromeretic principle. The study of various types of densities showed higher packability of MA spherical crystals compared with MA powder. The spherical crystals had an angle of repose that was lower than that of MA powder. This result suggests that the spherical crystals have excellent flow properties. The spherical crystals had greater wettability than did the MA powder, as indicated by the lower contact angle and surface tension. This result may have been caused by the lower crystallinity of agglomerated crystals as compared with the bulk drug. Spherical crystallization also increased the aqueous solubility of MA (see Table II).
[FIGURE 3 OMITTED]
Slugging was required to make a coherent tablet from MA powder, whereas spherical crystals formed a coherent tablet after one compression. Thus the spherical crystals are directly compressible, which could be a result of the new clear surface formed during compression.
A hardness study of tablets showed that the tablets prepared from spherical crystals had greater mechanical strength than those prepared from the powder (see Table III). This may be a result of the stronger bonds formed between newly formed crystals of agglomerates. A friability study showed lower friability of the tablets prepared from the spherical crystals, possibly owing to the better compaction of the spherical crystals. The solubility study showed that spherical crystals have good solubility in water as well as in other solvents. This increase in solubility may be a result of the decreased crystallinity and increased wettability of the agglomerates of crystals. Disintegration tests showed that the tablets of spherical crystals disintegrated more rapidly than did the tablets made from simple crystals of MA. Figure 4 shows that the dissolution rate of tablets of spherical crystals was higher than that of tablets made from simple MA, owing to the greater wettability of the spherical crystals.
[FIGURE 4 OMITTED]
Chemical stability studies showed that a plot of log (percent residual drug) versus time resulted in a straight line, which indicates that degradation followed first-order kinetics. The shelf life of tablets stored in the dark was 404 days for tablets made from MA spherical crystals and 397 days for tablets made from MA powder (see Figure 5). It is evident from the chemical stability data that the spherical crystals of MA did not show much degradation. When subjected to chemical stability studies at room temperature for a period of three weeks in light, both powder MA and spherical crystals of MA showed slightly more degradation as compared with those kept in the dark. A solution of MA powder, when kept in light at room temperature for a period of three weeks, showed 44% degradation, whereas a solution of spherical crystals of MA showed ~35% degradation (see Figure 6).
[FIGURE 6 OMITTED]
Conclusion
Spherically agglomerated crystals of mefenamic acid were successfully prepared for direct tableting using the spherical crystallization technique. The micromeritic properties of the agglomerated crystals such as flowability, packability, and compactibility were better than those of MA powder. This resulted in successful direct-tableting material without capping under a practical compression speed.
Acknowledgements
The authors express their gratitude to the electron microscopy division, Department of Anatomy, AIIMS, New Delhi, for the SEM studies and to Dr. H.S. Gout Vishwavidyalaya, Sagar (M.P.), the head of the Department of Pharmaceutical Sciences, for the use of the department's facilities. We also express our thanks to UGC, New Delhi, for providing a junior research fellowship to one of the authors involved in this research.
References
(1.) A.R. Paradkar, K.R. Mahadik, and A.P. Pawar, "Spherical Crystallization a Novel Particle Design Technique," Indian Drugs 31, (6), 283-299 (1998).
(2.) Y. Kawashima et al., "The Development of an Emulsion-Solvent-Diffusion Preparation Method of Agglomerated Crystals for Direct Tableting and Evaluation of the Compressibilities," J. Soc. Powder Technol. Japan 26, 659-665 (1989).
(3.) Y. Kawashima et al., "Improved Static Compression Behaviors and Tabletabilities of Spherically Agglomerated Crystals Produced by Spherical Crystallization Technique with a Two-Solvent System," Pharm. Res. 12 (7), 1040-1044 (1990).
(4.) "Micromeretics," in Physical Pharmacy, A. Martin, P. Bustamante, and A.H.C. Chum, Eds. (Waverly International, Maryland, 4th ed., 1995), pp. 492-522.
(5.) A.R. Gennaro, Ed., Remington's Pharmaceutical Science, (Mack Publishing Company, Pennsylvania, 18th ed., 1990), pp. 1615-1645.
(6.) G.S. Banker and N.R. Anderson, "Tablets," in The Theory and Practice of Industrial Pharmacy, L. Lachman, H.A. Lieberman, and J.L. Kaning, Eds. (Varghese Publishing House, Bombay, Indian Reprint, 3rd ed., 1987), pp. 296-302.
(7.) Pharmacopoeia of India, published by controller of publication, Delhi, 1996, Vol. 2, A-145.
Please rate this article.
On the Reader Service Card, circle a number:
342 Very useful and informative
343 Somewhat useful and informative
344 Not useful or informative
Your feedback is important to us.
Sulekha Bhadra, Manoj Kumar, Sunil Jain, Shikha Agrawal, and G.P. Agrawal *
* To whom all correspondence should be addressed.
Sulekha Bhadra, Sunil Jain, and Shikha Acjrawal are doctoral candidates; Manoj Kumar is a lecturer; and G.P. Agrawal, PhD, is a professor of pharmaceutics, all at the Department of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar, MP, 470003, India, tel. +91 7582 221632, fax +91 7582 222163, bhadrasb28@yahoo.com
COPYRIGHT 2004 Advanstar Communications, Inc.
COPYRIGHT 2004 Gale Group