PURPOSE: Prognosis of patients with pulmonary metastases remains poor with a 5-year survival of approximately 20-40% after complete surigical resection. Isolated lung perfusion (ILuP) is a promising surgical technique to deliver high-dose chemotherapy with minimal systemic toxicity, however exact pharmacokinetics of melphalan (MN) during ILuP remain unclear. An extension trial of a previous reported phase-I clinical trial of IluP with MN combined with pulmonary metastasectomy for resectable lung metastases was conducted to perform pharmacokinetic analysis.
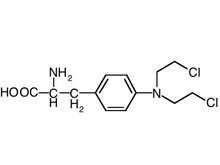
METHODS: From May 2001 to December 2004, 23 patients underwent ILuP with MN in increasing doses of 15, 30, 45 and 60 mg melphalan at 37[degrees]C and 42[degrees]C consecutively, followed by surgical resection of lung metastases. The pharmacokinetics of MN during ILuP were investigated using concentrations in tumour, lung tissue, perfusate and plasma with different perfusate drug concentrations and perfusion temperatures.
RESULTS: In total, 29 procedures of ILuP with complete metastasectomy were performed. High concentrations of MN were recorded in the perfusate in comparison to low systemic concentrations. Although there was a trend to correlation between the averaged MN concentrations in the perfusate after initiation of the procedure and the dose of MN at the start of the procedure, no significant correlation between perfusate and lung tissue or tumour MN concentrations could be recorded due to large variability in MN concentrations and the small number of patients per dose level.
CONCLUSION: ILuP with melphalan resulted in high-dose local chemotherapy with minimal systemic concentrations. In this study, no significant correlation between perfusate MN concentrations and tumour or lung tissue MN concentrations could be observed.
CLINICAL IMPLICATIONS: The absence of correlation between the perfusate and tumour or lung tisssue concentrations justifies further investigation of the pharmacokinetics of drugs during IluP in order to be able to account for observed variability.
DISCLOSURE: Marco Grootenboers, None.
Marco J. Grootenboers MD * Jeroen M. Hendriks PhD Wim J. van Boven MD Catherijne A. Knibbe PharmD Paul E. Van Schil PhD Franz M. Schramel PhD St. Antonius Hospital, Nieuwegein, Utrecht, Netherlands
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group