Abstract
The objective of this study was to evaluate the physical and chemical stability of undiluted palonosetron 50 µg/mL as the hydrochloride with fluorouracil 16 mg/mL, and with gemcitabine 10 mg/mL as the hydrochloride, in 5% dextrose injection during simulated Y-site administration.
Triplicate test samples were prepared by admixing 7.5 mL of palonosetron hydrochloride with 7.5 mL of the fiuorouracil and gemcitabine hydrochloride admixtures. Physical stability was assessed by using a multistep evaluation procedure that included both turbidimetric and particulate measurements as well as visual inspection. Chemical stability was assessed by using stability-indicating high-performance liquid chromatographic analytical techniques that determined drug concentrations. Evaluations were performed initially upon mixing and 1 and 4 hours after mixing.
The samples were clear and colorless when viewed in normal fluorescent room light and when viewed with a Tyndall beam. Measured turbidity remained unchanged; particulate content was low and changed little. High-performance liquid chromatographic analysis revealed that palonosetron hydrochloride, fluorouracil, and gemcitabine hydrochloride remained stable throughout the 4-hour test with no drug loss.
Palonosetron hydrochloride is physically compatible and chemically stable with fluorouracil and with gemcitabine hydrochloride during simulated Y-site administration.
Introduction
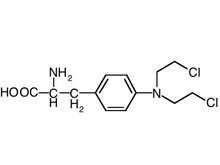
Palonosetron hydrochloride (HCl) (Aloxi) is a selective 5-HT3 receptor antagonist approved for use in the prevention of chemotherapy-induced nausea and vomiting.1-3 Palonosetron HCl has been demonstrated to be stable and compatible with several common infusion solutions, including 5% dextrose injection.4 However, palonosetron HCl injection may be administered with many other drugs by simultaneous or sequential Y-site administration, including fluorouracil and gemcitabine HCl. (The stability of palonosetron HCl with these other drugs needs to be determined as well.)
The purpose of this study was to evaluate the physical and chemical stability of undiluted palonosetron 50 pg/mL as the HCl with fluorouracil 16 mg/mL, and with gemcitabine 10 mg/mL as the HCl in 5% dextrose injection during simulated Y-site administration.
Methods
Materials
Palonosetron HCl injection 50 µg/mL, 2-mL vials (Lot HPA003) was supplied by MGI PHARMA, Inc. (Bloomington, Minnesota). Fluorouracil injection 50 mg/mL, 10-mL vials (Lot 121133, American Pharmaceutical Partners, Irvine, California) and gemcitabine HCl injection 200-mg vials (Lot 7RF96M, Eli Lilly and Company, Indianapolis, Indiana) were obtained commercially. The infusion solution, 5% dextrose injection (Lot PS134148, Baxter Healthcare, Deerfield, Illinois) in polyvinylchloride bags, was also obtained commercially. Palonosetron HCl reference standard (Batch 21000567, Helsinn Chemicals SA, Lugano, Switzerland) was supplied by MGI PHARMA, Inc., and was used without further purification. Reference standards for fluorouracil (Lot G-2, United States Pharmacopeial Convention, Inc., Rockville, Maryland) and gemcitabine (Lot 151GS4, Eli Lilly and Company) were obtained from the specified suppliers. The acetonitrile and other mobilephase components were high-performance liquid chromatographic (HPLC) grade. The water used was also HPLC grade (Milli-Q Plus; Millipore Corporation, Bedford, Massachusetts) and was prepared immediately before use.
Alien et al reported that the mixing of an intravenous fluid in an administration set with a secondary additive through a Y-injection site occurs in a 1:1 ratio.5 To simulate this inline mixing, triplicate samples were prepared by mixing 7.5 mL of undiluted palonosetron 50 µg/mL as the hydrochloride with 7.5 mL of fluorouracil 16 mg/mL, and separately with 7.5 mL of gemcitabine 10 mg/mL as the hydrochloride, in colorless 15-mL borosilicate glass screw-cap culture tubes (Kimble, Division of Owens-Illinois, Toledo, Ohio) with polypropylene caps (Kimble) as described elsewhere.'1 The individual drug solutions were filtered through 0.22-pm filters (Millex-GS; Millipore Corporation) into the tubes. All manipulations were carried out in a Class 100 biological safety cabinet.
Physical Stability
The physical stability of the admixtures was assessed by visual examination and by measuring turbidity and particle size and content.6-8 The sample tubes had been previously triple-washed in HPLC-grade water and dried. To minimize the effects of scratches and imperfections in the glass, a thin layer of silicone oil was applied to the tube exteriors. Visual examinations were performed in normal diffuse fluorescent room light with the unaided eye and using a high-intensity monodirectional light (Tyndall beam; DolanJenner Industries, Woburn, Massachusetts).
The turbidity of each sample was measured by using a color-correcting turbidimeter (Model 2100AN; Hach Company, Loveland, Colorado). Triplicate determinations were made on each of the samples. After 4 hours, a light obscuration particle sizer/counter (Model 9703; Hiac-Royco, Division of Pacific Scientific Company, Grants Pass, Oregon) was used to quantify particle content of the samples in the 1.04-µm to 112-µm range (the validated detection limits of the particle sizer/counter) and to verify the absence of unacceptable amounts of microparticulates. Triplicate determinations were made on these samples. Physical instability was defined as visible paniculate matter, haze, color change, or a change (increase or decrease) in measured turbidity of 0.5 nephelometric turbidity unit (NTU) or more.6-8
High-Performance Liquid Chromatographic Analysis
The drug concentrations were determined by using stabilityindicating HPLC assay methods. The details of the analytical methods used in this study are cited in Table 1. The palonosetron HCl and gemcitabine HCl analytical methods were provided by the drug manufacturers.9-11 The fluorouracil analytical method was developed and validated in our laboratory. The high-performance liquid chromatograph used for analysis of palonosetron HCl was an LC Module 1 (Waters Corporation, Milford, Massachusetts). It consisted of a multisolvent delivery pump, autosampler, and ultraviolet light detector. The high-performance liquid chromatograph used for analysis of fluorouracil and gemcitabine consisted of an Alliance 2690 separations module (Waters Corporation) and a Waters 2480 dual wavelength detector (Waters Corporation). The systems were controlled and integrated by a personal computer with chromatography management software (Millennium 32 Chromatography Manager, Waters Corporation). Triplicate HPLC determinations were performed on triplicate samples of each test admixture solution.
The analytical methods for each of the drugs were demonstrated to be stability indicating by accelerated degradation. The sample solutions were mixed with 1 N sodium hydroxide, 1 N hydrochloric acid, and 3% hydrogen peroxide, and were subjected to heating. Loss of the intact drugs was observed, and the degradation product peaks or other drug peaks did not interfere with the peak of the intact subject drug.
The initial concentrations of palonosetron as the HCl, fluorouracil, and gemcitabine as the HCl were defined as 100%, and subsequent sample concentrations were expressed as a percentage of the initial concentration. Acceptable stability of the drug was defined as not less than 90% of the initial drug concentration remaining in the admixtures.
Results and Discussion
All of the admixtures were initially clear and colorless in normal fluorescent room light and when viewed with a Tyndall beam. Both the palonosetron HCl-fluorouracil samples and the palonosetron HCl-gemcitabine HCl samples were essentially without haze, having measured turbidities of about 0.15 NTU or less. Changes in turbidity for the samples were minor throughout the study, generally less than 0.03 NTU. Measured particulates of 10 pm or larger were few in number in all samples throughout the observation periods. The admixtures remained colorless throughout the study.
The results of the HPLC analysis for each of the test drugs are shown in Tables 2 and 3. No loss of concentration of any of the drugs was observed.
Conclusion
Palonosetron HCl is physically and chemically stable with fluorouracil and gemcitabine HCl for 4 hours, establishing acceptability for Y-site administration.
References
1. Aloxi [package insert]. Bloomington, MN: MGI PHARMA, Inc., July, 2003.
2. Stacher G. Palonosetron (Helsinn). Curr Opin Investig Drugs 2002; 3(10): 1502-1507.
3. Eglen RM, Lee CH, Smith WL et al. Pharmacological characterization of RS 25259-197, a novel and selective 5-HT3 receptor antagonist, in vivo. Br J Pharmacol 1995:114(4): 860-866.
4. Trissel LA, Xu QA. Physical and chemical stability of palonosetron HCI with 4 infusion solutions. Ann Pharmacother2004; 38(10): 1608-1611.
5. Alien LV Jr, Levinson RS, Phisutsinthop D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am J Hosp Pharm 1977; 34(9): 939-943.
6. Trissel LA, Bready BB. Turbidimetric assessment of the compatibility of taxol with selected other drugs during simulated Y-site injection. Am J Hosp Pharm1992;49(7):1716-1719.
7. Trissel LA, Martinez JF. Turbidimetric assessment of the compatibility of taxol with 42 other drugs during simulated Y-site injection. Am J Hosp Pharm 1993; 50(2): 300-304.
8. Trissel LA, Martinez JF. Physical compatibility of melphalan with selected drugs during simulated Y-site administration. Am J Hosp Pharm 1993; 50(111:2359-2363.
9. [No author listed.] HPLC assay of gemcitabine hydrochloride (LY188011111-HCI) in bulk, lyophilized vials, and solutions. Indianapolis, IN: EIi Lilly and Company; March 17, 1997.
10. Xu Q, Zhang Y, Trissel LA. Physical and chemical stability of gemcitabine hydrochloride solutions. J Am Pharm Assoc 1999; 39(4): 509-513.
11. [No author listed.] Palonosetron HCIIV injection 0.05 mg/mL and 0.15 mg/mLend product test procedure. Minneapolis, MN: MGI PHARMA, Inc.; (Undated).
Lawrence A. Trissel, BS, RPh, FASHP
Yanping Zhang, BS
Clinical Pharmaceutics Research
Division of Pharmacy
The University of Texas M. O. Anderson Cancer Center
Houston, Texas
Acknowledgment
This study was supported by a grant (LS2003-00008893AG01) from MGI PHARMA, Inc., Bloomington, Minnesota.
Address correspondence to Lawrence A. Trissel, BS, RPh, FASHP, Division of Pharmacy, Box 90, The University of Texas M. D. Andersen Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030.
Copyright International Journal of Pharmaceutical Compounding Jul/Aug 2005
Provided by ProQuest Information and Learning Company. All rights Reserved