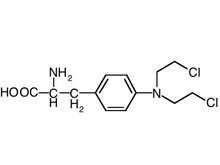
Caradonna S, Jacobe H. Arch Dermatol 2004 Mar; 140(3):277-80.
The authors present a case of a 39-year-old female with a history of scleromyxedema for about one year. The patient's only complaint was of limited joint mobility. A skin biopsy was performed on the patient's skin to evaluate the sclerotic plaques on her body, and her results showed dermal thickening with displacement of collagen bundles by mucin, with spindled fibroblasts consistent with scleromyxedema.
The patient was started on prednisone and melphalan with no improvement. She was then changed from melphalan to cyclophosphamide and continued on prednisone with minimal change. Later on a trial of cladribine was instituted with no success and afterwards the patient was maintained on prednisone with no improvement. After seven years of various treatments, she was treated with thalidomide 100 mg/d and after just six months of therapy she started to notice increase mobility and less skin thickness. A repeat biopsy at one year of thalidomide therapy demonstrated a decrease in mucin.
JDD ARTICLE EVALUATION
Scleromyxedema is a disease of adults that mainly affects patients of 30-50 years of age. The pathogenesis is unknown and treatment modalities are sparse and unsuccessful. In the past melphalan has been known to clear the disease but long term use is associated with hematologic malignancies. Thalidomide offers hope to patients with scleromyxedema, its mechanism of action is unknown but it is believed that it acts through TNF alpha inhibition. In the past it has been shown to be effective in neutrophilic dermatosis and in animal models it may represent a useful anti-neoplastic agent.
Although an interesting case report, we would hope for a follow up studies to compare thalidomide with other treatment modalities in a double-blinded randomized study. All in all, this represents a new regimen, albeit off-label, for the treatment of scleromyxedema.
WASHINGTON WHISPERS APPEARS IN EACH ISSUE AND PROVIDES A SUMMARY AND CRITICAL EVALUATION OF THE LATEST DRUG TRIALS, STUDIES, AND REACTIONS AVAILABLE TO THE MEDICAL COMMUNITY, AS COLLATED FROM A WEALTH OF INDUSTRY SOURCES.
COPYRIGHT 2004 Journal of Drugs in Dermatology, Inc.
COPYRIGHT 2005 Gale Group