Abstract
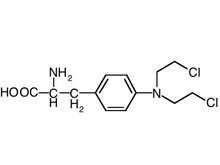
The objective of this study was to evaluate the physical and chemical stability of treprostinil (as sodium) injections in concentrations of 1, 2.5, 5 and 10 mg/mL packaged in MiniMed plastic syringe pump reservoirs.
Test samples of treprostinil (as sodium) injections having concentrations of 1, 2.5, 5 and 10 mg/mL were packaged as 3 mL of drug solution in 3-raL MiniMed plastic syringe pump reservoirs, sealed with plastic tip caps and stored at -20°C, 4°C, 23°C and 37°C for 60 days. Evaluations for physical and chemical stability were performed initially and throughout the storage periods. Physical stability was assessed using visual observation in normal room light and using a high-intensity monodirectional light beam. In addition, turbidity and particle content were measured electronically. Chemical stability of the drug was evaluated by using a stability-indicating high-performance liquid chromatographic analytical technique.
All samples of treprostinil (as sodium) injection remained free of visible precipitation throughout the study. Little or no change in haze level and in particulates of > or =10 µm was found. Changes in treprostinil concentration were found to be small; treprostinil sodium concentrations were found to be 95% or greater over 60 days at all temperatures studied.
Treprostinil (as sodium) injections at concentrations ranging from 1 to 10 mg/mL can be packaged in MiniMed plastic syringe reservoirs, stored and shipped with little or no loss of drug stability.
Introduction
Treprostinil sodium (Remodulin, United Therapeutics Corporation, Research Triangle Park, North Carolina) is indicated for the treatment of pulmonary arterial hypertension in appropriate patients by continuous subcutaneous infusion using an appropriate infusion delivery system. The drug is formulated with an antimicrobial preservative (metacresol) and is packaged in multiple-use vials.1 However, the stability information available in the manufacturer's labeling1 is limited; the maximum noted is 72 hours under use conditions at 370C. This abbreviated stability information is insufficient to support prepackaging of the injection in the syringe reservoirs of a suitable MiniMed delivery system, storage under appropriate conditions and possibly shipping to use sites.
The objective of this study was to evaluate the physical and chemical stability of treprostinil (as sodium) injections in concentrations of 1, 2.5, 5 and 10 mg/mL. The test injections were packaged as 3 m L of the treprostinil sodium injections in 3-mL plastic MiniMed syringe reservoirs and stored frozen, refrigerated, at room temperature and at body temperature for 60 days.
Methods
Materials
Treprostinil (as sodium) injections (Lots 802324B [1 mg/mL], 802163A [2.5 mg/mL], 802278F [5 mg/mL], 802138G [10 mg/mL], United Therapeutics Corporation) were obtained from the manufacturer. Treprostinil reference standard (Lot UT15-000901, United Therapeutics Corporation) was also obtained from the manufacturer and was used without further purification. The acetonitrile and other mobile phase components were high-performance liquid Chromatographie (HPLC) grade. The water used was also HPLC grade and was prepared immediately before use.
Preparation and Storage of Sample Solutions
Treprostinil (as sodium) injections of 1, 2.5, 5 and 10 mg/mL were packaged as 3 mL of injection in 3-mL plastic syringe reservoirs (Lot AJ0402541, Medtronic MiniMed, Northridge, California) and sealed with plastic-tip caps (Red Cap, Burron Medical Inc., Bethlehem, Pennsylvania). Three replicate syringes of each concentration for each evaluation time point were stored at temperatures -20°C, 4°C, 23°C and 37°C over 60 days. Samples stored in the refrigerator and freezer as well as those in the elevated temperature incubator were protected from light. Samples stored at room temperature were left exposed to normal fluorescent room light for 18 hours each day.
Physical Stability Assessment
The physical stability of the trcprostinil sodium injections was assessed by visual examination and by measuring turbidity and particle size and content.2-4 Five-milliliter samples pooled from the three syringes of each of the treprostinil concentrations were transferred into 15-mL borosilicate glass culture tubes (Kimble, Division of Owens-Illinois, Toledo, Ohio) with polypropylene screw caps (Kimble). Prior to sample testing the tubes were triple-washed in HPLC-grade water and dried. Samples were evaluated initially and after storage for 60 days at each temperature condition.
To minimize the effects of scratches and imperfections in the glass, a thin layer of silicone oil was applied to the exteriors of the tubes. Visual examinations were performed at each of the time points in normal diffuse fluorescent room light with the unaided eye and using a high-intensity monodirectional light (Tyndall beam, Dolan-Jenner Industries, Woburn, Massachusetts).4
The turbidity of each sample was measured using a colorcorrecting turbidimeter (Model AN2100, Hach Company, Loveland, Colorado). The particle content of the samples was quantified using a light obscuration particle sizer/counter (Model 8003, Hiac-Royco, Division of Pacific Scientific Company, Silver Spring, Maryland) to determine particle content in the size range of 1.04-112 µm (the validated detection limits of the particle sizer/counter). Triplicate determinations were made on each of the samples.
Physical stability was defined as the absence of visible change (paniculate matter, haze or color) or measured change (an increase in measured turbidity of 0.5 nephelometric turbidity unit [NTU]) for clear drugs like treprostinil sodium injection that exhibit no inherent haze.2-4
Chemical Stability Evaluation
The analytical method used in this study was developed to conduct this project and is described in Table 1. The HPLC consisted of a multisolvent delivery pump, ultraviolet (UV) detector and autosampler in one unit (LC /Module 1, Waters Corporation, Milford, Massachusetts) and a Spherisorb CN analytical column (Alltech Corporation, Deerfield, Illinois). The system was controlled and integrated by a personal computer with chromatography management software (Millennium 32, Chromatography Manager, Waters Corporation). The samples were diluted with mobile phase to a nominal treprostinil concentration of 0.05 mg/mL. Triplicate HPLC determinations were performed on each of the triplicate samples for a total of nine assays of each test combination at each time point. Aliquots of 1 mL were removed from each of three replicate syringes for the evaluation of chemical stability after storage at all temperatures for 7, 14, 30 and 60 days. Analysis was performed on the aliquots immediately after being removed from the samples.
The HPLC analytical method was validated to be stabilityindicating by accelerated degradation. Using this HPLC] method, intact treprostinil eluted at about 8.0 minutes while the nietacresol preservative eluted at 4.8 minutes. Accelerated decomposition was performed by heating sample solutions and exposure to 1 N hydrochloric acid, 1 N sodium hydroxide and also 3% hydrogen peroxide. The formation of multiple new peaks was observed at about 2.S through 4.0 minutes, along with a reduction in the intact drug peak. The degradation product peaks did not interfere with the intact treprostinil or metacrcsol peaks.
The initial concentrations of treprostinil (as sodium) in the samples were denned as 100%, and subsequent sample concentrations were expressed as a percentage of the initial concentration. Chemical stability of the treprostinil sodium was defined as not less than 90% of the initial drug concentration remaining in the samples.
Results and Discussion
All of the samples were initially clear and colorless in normal fluorescent room light. The injections were essentially without haze, having measured turbidities between 0.1 and 0.25 NTU.
All samples of the treprostinil sodium injections remained colorless and free of visible precipitation throughout the study. Changes in measured turbidity were minor throughout the study with none exceeding 0.1 NTU. Furthermore, there was no evidence of microprecipitate development. By electronic evaluation, total particulates were found to be near the background amounts with no increase in particulates > or =10 µm in size.
Relatively little change in the concentration of treprostinil sodium occurred in any of the samples at any storage temperature throughout the study. The analytical results demonstrate that the treprostinil (as sodium) remained intact. Treprostinil concentrations were found to be 95% or greater over 60 days at all storage temperatures (Table 2). Additionally, the areas of the metacresol peaks remained essentially unchanged throughout the study.
The chemical stability results are consistent with another study that reported the stability of treprostinil sodium in aqueous solution.5 This present study has demonstrated that treprostinil (as sodium) injections at concentrations ranging from 1 to 10 mg/mL packaged in MiniMed plastic syringe pump reservoirs, stored frozen at -20°C, refrigerated, at room temperature, and at body temperature remained stable for 60 days.
Conclusion
Treprostinil (as sodium) injections at concentrations of 1, 2.5, 5 and 10 mg/mL can be packaged in MiniMed plastic syringe pump reservoirs, stored and shipped with little or no loss of drug occurring.
References
1. Remodulin [package insert). Research Triangle Park, NC: United Therapeutics Corporation, 2002.
2. Trissel LA, Bready BB. Turbidimetric assessment of the compatibility of taxol with selected other drugs during simulated Y-site injection. Am J Hasp Pharm m2; W: 1716-1719.
3. Trissel LA, Martinez JR Turbidimetric assessment of the compatibility of taxol with 42 other drugs during simulated Y-site injection. Am J Hasp Pharm 1993; 50: 300-304.
4. Trissel LA, Martinez JF. Physical compatibility of melphalan with selected drugs during simulated Y-site administration. Am J Hosp Pharm 1993; 50:2359-2363.
5. Phares KR, Weiser WE, Miller SP et al. Stability and preservative effectiveness of treprostinil sodium after dilution in common intravenous diluents. Am J Health Syst Pharm 2003; 60: 916-922.
Quanyun A. Xu, PhD
Lawrence A. Trissel, BS, RPh, FASHP
Clinical Pharmaceutics Research
Division of Pharmacy
The University of Texas
M. D. Andersen Cancer Center
Houston, Texas
Lien Pham, PharmD
Priority Healthcare Corporation
Lake Mary, Florida
This study was supported by a grant (LS2003-00008324CG01) from Priority Healthcare Corporation, Lake Mary, Florida.
Address correspondence to: Lawrence A. Trissel, IiS, RPh, VASHP, Division of Pharmacy, Box 90, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, Texas 77030.
Copyright International Journal of Pharmaceutical Compounding May/Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved