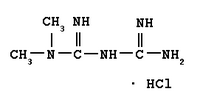
Metformin
Metformin (Glucophage®, Fortamet®, Riomet®) is an anti-diabetic drug from the biguanide class (its other members are the withdrawn agents phenformin and buformin). more...
Uses
The main use for metformin is for the treatment of diabetes mellitus, especially when it is concomitant with obesity and insulin resistance.
It is also being used increasingly in polycystic ovary syndrome (PCOS) and non-alcoholic steatohepatitis, two other diseases that feature insulin resistance; these indications are still considered experimental.
Metformin is the only anti-diabetic drug that has been proven to reduce the complications of diabetes, as evidenced in a large study of overweight patients with diabetes (UKPDS 1998).
Metformin is often prescribed to type 2 diabetes patients in combination with rosiglitazone maleate. This drug actively reduces insulin resistance, complementing the action of the metformin. In 2002, the two drugs were combined into a single product, Avandamet, marketed by GlaxoSmithKline. . In 2005, all current stock of Avandamet was seized by the FDA and removed from the market. This was due to problems at the manufacturing plants, not to any medical issues resulting from the drugs use. The drug pair continued to be prescribed separately in the absence of Avandamet itself, which was readily available by the end of that year.
Mechanism of action
Despite its therapeutic benefits, the mechanism of action of metformin is uncertain. Its mode of action appears to be reduction of hepatic gluconeogenesis; the "average" person with type 2 diabetes has three times the normal rate of gluconeogenesis. Metformin treatment reduces this by one third to two thirds. It is has been shown that metformin also decreases intestinal absorption of glucose. A third mechanism is that metformin improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Zhou et al (2001) showed that metformin stimulates the hepatic enzyme AMP-activated protein kinase.
Side-effects
The most serious side effect of metformin is lactic acidosis. However, this complication is rare if the contra-indications are followed, as it seems limited to those with impaired liver and/or kidney function.
Phenformin was withdrawn because of an increased risk of lactic acidosis (up to 60 cases per million patient-years). In recent studies it was revealed that, as long as it is not prescribed to patients who are at risk, metformin is much safer, and the risk of lactic acidosis approximates that of people who are not on the medication (Salpeter SR et al 2003).
The most common side effect of metformin is gastrointestinal upset. This includes diarrhea, cramps, nausea and vomiting. In a clinical trial of 286 subjects, 53.2% of the 141 who were given Metformin IR (as opposed to placebo) reported diarrhea, and 25.5% reported nausea/vomiting (source: Drug Facts & Comparisons 2005).
Read more at Wikipedia.org