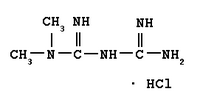
The recent Diabetes Prevention Program (DPP) reported that the incidence of diabetes in individuals with impaired glucose tolerance (IGT) was reduced by 58% when lifestyle modifications were followed and by 31% with metformin therapy as compared with placebo after a mean duration of 2.8 years of intervention. At the completion of the DPP and in order to determine whether the observed effect of metformin on the development of diabetes lasted after metformin was withdrawn, researchers tested participants taking metformin or placebo who had not developed diabetes with a repeat oral glucose tolerance test (OGTT) after a 1- to 2-week washout period during which medication was withheld.
Specifically, researchers asked the following questions: 1) Among those who did not convert to diabetes during the DPP and before washout, do the metformin and placebo groups differ in the rate of conversion to diabetes after the washout? 2) If the diabetes conversions during washout were combined with the conversions to diabetes during the trial, do the metformin and placebo groups differ in the proportion of participants who have converted to diabetes?
The subjects remained masked to their treatment assignments, were scheduled for a washout visit, and were asked to discontinue the study medication one week before the washout visit. Because of logistics of furthering the study, the actual time of drag withdrawal ranged from one to two weeks. The visit consisted of an OGTT conducted according to the DPP protocol. Following the OGTT, participants were asked to remain off the medication until they were notified whether a diabetes confirmation visit was necessary. The odds ratios for conversion to diabetes at washout for metformin versus placebo were estimated using the Mantel-Haenszel adjustment for the year of randomization. There were 1274 participants who were involved in the washout study and 52 who were not involved because they had already been diagnosed with diabetes. Prior to the washout, the odds of diabetes in the metformin group was lower than that in the placebo group (odds ration 0.66, 95% CI 0.54-0.82, P less than 0.001). Following the washout, diabetes was somewhat more frequently diagnosed in the metformin participants (1.49, 0.93-2.38, P=0.098). Combining diabetes conversions during the DPP and during the washout, diabetes was diagnosed significantly less frequently in the metformin than the placebo group (0.75, 0.62-0.92, P=0.005).
This data demonstrates that metformin decreases the risk of diabetes by 31% in this at risk population. The washout study indicates that 26% of this effect can be accounted for by a pharmacological effect of metformin that did not persist when the drug was discontinued.
W. Knowler, E. Barrett-Connor, S. Fowler, et al. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program, Diabetes Care (26:977-980, April, 2003). Correspondence: Mark E. Molitch, MD, Diabetes Prevention Program Coordinating Center, Biostatistics Center, George Washington University, 6110 Executive Blvd., Suite 750, Rockville, MD 20852. E-mail: dppmail@biostat.bsc.gwu.edu
COPYRIGHT 2003 Frost & Sullivan
COPYRIGHT 2003 Gale Group