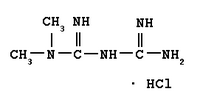
Some doctors prescribe metformin, the most widely used pharmaceutical treatment for patients with type 2 diabetes and with congestive heart failure, kidney dysfunction, or both, despite clear warnings on drug packaging not to do so, a new study indicates.
At the University of North Carolina at Chapel Hill, it was noted that 22 of 100 randomly selected patients who were receiving metformin had one or both of the contraindications.
"Our findings are consistent with several recent studies in Europe that documented similar rates of inappropriate metformin prescribing," said Dr. Cheryl Horlen, who conducted the research as a pharmacy resident at the University. "We are concerned that these patients may be at risk of a serious medical condition known as lactic acidosis, which can be life-threatening."
In the first 14 months after its release in the U.S., the Food and Drug Administration (FDA) received reports of 47 confirmed cases of lactic acidosis associated with metformin, she said. The mortality rate was 42 percent. In the absence of contraindications, the drug is considered safe and effective.
A report on the study appears as a letter in The Journal of the American Medical Association (May 15, 2002).
"We performed a retrospective review of hospital charts of patients receiving metformin through the outpatient pharmacy at an academic medical center," Dr. Horlen said. "We found 241 patients who had two or more prescriptions for metformin within a 9-month period and then randomly selected 100 of those for the chart review."
Fourteen of the patients had congestive heart failure only, five had kidney dysfunction, and three had both. The mean age of patients was 60 years; half were women and half were black.
"Only two patients had documentation in their medical records that providers had considered the metformin contraindications," she said. "Because our assessment of the prevalence of contraindications to metformin use relied on a chart review, it might have underestimated the frequency of contraindications. It also was difficult to determine whether providers were aware they were prescribing metformin against what is called a black box warning, which is specific warning with a black border around it to attract attention."
Physicians and other health care providers should think about improving documentation of the risk of lactic acidosis and should offer counseling for patients who receive the drug, she said. Considering the new study and the European results, there is no reason to believe that metformin-prescribing practices differ much in other parts of the U.S.
"Although this was a relatively small study, the fact that almost one in four prescriptions for metformin were being prescribed against the black box warning may be significant," said Dr. Russell Rothman, a Robert Wood Johnson Clinical Scholar at the University. "With more than 25 million prescriptions of metformin written a year, the potential for adverse effects needs to be taken very seriously. More research will need to be performed to ensure that patients are receiving optimal care."
COPYRIGHT 2003 Vegetus Publications
COPYRIGHT 2003 Gale Group