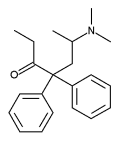
Methadone is a synthetic opioid with potent analgesic effects. Although it is associated commonly with the treatment of opioid addiction, it may be prescribed by licensed family physicians for analgesia. Methadone's unique pharmacokinetics and pharmacodynamics make it a valuable option in the management of cancer pain and other chronic pain, including neuropathic pain states. It may be an appropriate replacement for opioids when side effects have limited further dosage escalation. Metabolism of and response to methadone varies with each patient. Transition to methadone and dosage titration should be completed slowly and with frequent monitoring. Conversion should be based on the current daily oral morphine equivalent dosage. After starting methadone therapy or increasing the dosage, systemic toxicity may not become apparent for several days. Some medications alter the absorption or metabolism of methadone, and their concurrent use may require dosing adjustments. Methadone is less expensive than other sustained-opioid formulations.
*********
Methadone significant is a synthetic opioid. Although structurally dissimilar to morphine (MS Contin), methadone has analgesic qualities. Because the high dosages used in preliminary testing of methadone caused substantial side effects, the drug was not used clinically for several years. (1) During the 1950s, methadone emerged as a treatment for opioid addiction and has remained the primary therapy for this condition for more than 40 years. Recently, methadone has been used to manage cancer pain and other chronic pain states. Its unique pharmacokinetics and pharmacodynamics make methadone a valuable option, but physicians should be aware of possible side effects.
Prescriptive Authority
Methadone is listed on schedule II of the Controlled Substances Act. Initially, its use was limited to "detoxification treatment" or "maintenance treatment" within U.S. Food and Drug Administration-approved narcotic addiction programs. (2) This restriction was removed in 1976; all physicians with appropriate Drug Enforcement Agency registration now are allowed to prescribe methadone for analgesia. (3) An indication, such as "for chronic pain," may be added to the written prescription to clarify its purpose. State laws vary regarding this documentation requirement. Not all pharmacies stock methadone because of its association with the treatment of heroin addiction.
Pharmacokinetics
Methadone is a highly lipophilic molecule that is suitable for a variety of administration routes. Approved for oral and intramuscular use, it also is used rectally, intravenously, subcutaneously, epidurally, and intrathecally. Oral methadone has a bioavailability close to 80 percent compared with 26 percent for morphine. (4) Methadone is absorbed rapidly from the stomach, with little absorption occurring beyond the pylorus. Following absorption, it is distributed to the brain, liver, kidneys, muscles, and lungs. Tissue binding predominates over binding to plasma proteins, and accumulation of the drug occurs in these tissues with repeated dosing. (5) Plasma concentrations are maintained by this peripheral reservoir. Methadone reabsorption from the tissues may continue for weeks after administration has ceased.
Methadone is metabolized in the liver with no active metabolites. It has an elimination half-life of about 22 hours, but metabolism varies in each person. (6) Unlike morphine, it usually is not necessary to adjust the dosage of methadone in patients with renal insufficiency. The duration of analgesia is approximately three to six hours when methadone therapy is initiated, and this duration typically extends to eight to 12 hours with repeated dosing. In a study of cancer patients, an average of 2.4 doses per day was required to maintain adequate pain control. (7) Because of its long half-life, plasma levels of methadone may take five to seven days to stabilize. By comparison, oral morphine has a half-life of two to four hours. The four- to six-hour duration of analgesia for oral morphine does not change with repeated dosing, and six or more doses may be required each day to maintain adequate pain control.
Pharmacodynamics
Methadone is a muopioid agonist. Analgesia and typical opioid side effects are the result of action at the muopioid receptor. Methadone has a mureceptor affinity similar to that of morphine but, with repeated dosing, its efficacy is greater than that of morphine. (8) There is no clear explanation for the brevity of analgesic effect in view of the long half-life. Methadone has nonopioid actions, including inhibition of the reuptake of monoamines (e.g., serotonin, norepinephrine) and inhibition of N-methyl-aspartate (NMDA) receptors-pharmacologic actions that result in additional analgesia. (8) Activation of the NMDA receptor can produce central sensitization (i.e., lowering central nervous system pain thresholds), so blocking this receptor may help prevent the development of tolerance. (9) In vitro studies have shown that morphine also will antagonize NMDA receptors but at concentrations eight to 16 times higher than required by methadone. (10) Beyond the initial titration, frequent or large dosage changes usually are not necessary with methadone.
Indications
Methadone has been studied as a therapy for cancer pain and other chronic pain states. (11,12) It is an appropriate replacement opioid when pain remains poorly controlled or when side effects of other opioids limit dosage escalation. Available data suggest that methadone is effective in relieving cancer pain and has a similar analgesic efficacy and side effect profile to morphine. (13) In a study of cancer patients with uncontrolled pain or significant side effects from opioids, 80 percent of patients reported improvement in pain control and reduction of adverse effects following transition to methadone. (14) It may be used in patients with morphine allergy because methadone is synthetic and offers no crossallogenicity. However, a 2004 Cochrane Review stated several considerations in evaluating trials of methadone for cancer pain. (13) The majority of studies reviewed involved single-dose comparisons or short-term use, which does not adequately represent clinical practice. Therefore, there is a highly significant danger that the trials do not reflect delayed adverse effects from methadone accumulation during chronic administration. The same review reported there is no trial evidence to support the proposal that methadone has a particular role in neuropathic pain of malignant origin. (13) Table 1 lists current indications for the use of methadone in the management of chronic pain.
Methadone Dosing
OPIOID-NAIVE PATIENTS
Although methadone is not a common first-line opioid, its use in opioid-naive patients may have some benefits. Its slow onset and long duration of effect can help avoid establishing the reward behaviors that can occur with fast-acting, short-duration opioids. In 2000, the College of Physicians and Surgeons of Ontario published a guideline for recommended methadone dosing. (15) In an opioid-naive patient, the recommended starting dosage is 2.5 mg orally every eight hours. In frail older patients, the starting dosage may need to be as low as 2.5 mg orally once per day. In the outpatient setting, incremental increases may be made every five to seven days, depending on the patient's response. (15)
OPIOID-TOLERANT PATIENTS
No single ratio is suitable for converting a specific dose of morphine into an equivalent dose of methadone. A 15-mg dose of oral morphine has the approximate analgesic equivalent of 10 mg of oral methadone; however, with repeated dosing, relatively small doses of methadone may have the analgesic efficacy of much larger doses of morphine. Most published narcotic equivalence charts report only single-dose equivalence. Systemic toxicity-including respiratory depression and death (in extreme cases)-can result from relying on these tables for chronic dosing because such reliance likely will result in a substantial overdose that may not be apparent for several days.
The following conversion plan relies on fixed doses administered at fixed intervals. Other available protocols use variable dosing intervals or stage the conversion process over the course of several days. (16,17)
Dose Conversion--Step 1. The first step in conversion to methadone therapy is to determine the daily oral morphine equivalent dose of the current opioids. The latter may include long-acting medications as well as short-acting medications used for breakthrough pain. The daily dosage of each opioid is multiplied by the ratio of equianalgesic doses, using morphine in the numerator and the current opioid in the denominator (Table 2). (18) These are summed, and the result is the daily oral morphine equivalent dose. If the current route of administration is not oral, the additional step of converting this route to the oral-equivalent dose is required. These conversion ratios usually are found in the package inserts of the associated products or on the manufacturers' Web sites. Figure 1 shows a sample calculation of the daily oral morphine equivalent dose from oxycodone (Oxycontin) and hydromorphone (Dilaudid).
Dose Conversion--Step 2. Studies consistently demonstrate that the conversion ratio of morphine to methadone depends on the daily oral morphine equivalent dosage (Figure 1). (19-21) Conversion ratios are shown in Table 3. (22) These ratios were based on records from patients who were rapidly converted from opioids to methadone over several days. Ratios then were developed by retrospectively comparing the oral morphine equivalent of the original opioid with the final methadone dosage. For a relatively small daily dosage of morphine (less than 100 mg), a ratio of 3 to 1 (33 percent) was proposed. The proportion of methadone decreases progressively to 20 to 1 (5 percent) for large daily dosages of morphine (more than 1,000 mg). (22) Application of these ratios tends to place an upper limit on the starting dose of methadone. For example, daily oral morphine dosages of 300 mg, 600 mg, 900 mg, and 1,200 mg all convert to 60 mg of methadone.
[TABLE 3 OMITTED]
In this second step, the daily oral morphine equivalent dosage is multiplied by the appropriate conversion ratio to arrive at the daily methadone dosage. One third of the calculated methadone dosage is used by the patient every eight hours (Figure 1).
TITRATION AND MONITORING
In opioid-tolerant patients, it may be necessary to provide rescue medication during the titration period. Methadone may be adjusted daily when continuous monitoring is available, and increases should be based on symptoms and the need for breakthrough medications. Incremental increases of 20 to 30 percent have been used safely in hospitalized patients. (23) Several days are required to reach steady-state plasma levels, so monitoring should continue after the last dosage increase to detect potential overdose. Contrary to expectations, toxicity occurs more frequently in patients previously exposed to high dosages of opioids. (24) Transition from high-dosage opioids may have to be completed in an inpatient setting with assistance from a pain specialist.
In the outpatient setting, methadone should be titrated cautiously, based on patient response and signs of toxicity. At-home transition to methadone can be safe even in older patients if follow-up is closely monitored. (25) Increases should not be made more frequently than every five to seven days, and the optimal incremental dosage increase is unclear; few studies support any specific protocol. During the titration phase, daily telephone progress reports by the patient, family members, home health nurses, or hospice personnel are recommended. Patients should be informed that several titrations might be necessary to reach optimal pain control.
As with any drug, when methadone therapy begins or dosages are changed, patients should be warned about the possible impairment of driving ability or other activities requiring focused concentration. Several days may be necessary before the blood levels stabilize and the full effects of methadone are appreciated.
Drug Interactions
A number of medications can change methadone's absorption, distribution, and metabolism. Methadone's absorption is mediated by gastric pH and P-glycoprotein (Pgp), a transport protein. Changes in gastric pH or the activity of Pgp brought about by certain medications (e.g., verapamil [Calan], quinidine) may change methadone absorption. (26,27) Methadone is metabolized principally by the CYP3A4 and CYP2D6 enzymes.6 Many medications interact with methadone via their effects on these enzymes, by inducing or inhibiting metabolism (Table 4). (6,8,26-28) Dosing adjustments may be required if medications are added to or eliminated from a patient's regimen. Analgesics with opioid-antagonist properties (e.g., buprenorphine [Subutex], butorphanol [Stadol], dezocine [Dalgan], nalbuphine [Nubain], nalorphine [Nalline], pentazocine [Talwin]) should not be used with methadone because they can displace methadone from muopioid receptors.
Cautions
Side effects associated with methadone are similar to those incurred with other muopioid agonists, including pruritus, nausea, constipation, confusion, sedation, and respiratory depression. Excess sweating (diaphoresis) and flushing are common with oral methadone dosing. Caution should be taken with initiation of therapy and dosage increases because severe toxicities may not become apparent for two to five days. In a study of patients converted to methadone therapy in an outpatient setting, 20 of 29 participants experienced some degree of toxicity, most frequently mild drowsiness, during initial titration. (29) Side effects such as sedation and respiratory depression are increased when methadone is combined with alcohol or other drugs. An Australian study (30) found benzodiazepines present in 74 percent of deaths related to methadone and urged particular caution when methadone was prescribed with benzodiazepines.
Cost
Methadone offers a cost savings over brand-name opioids in sustained-release or transdermal formulations. (31) In the United States, methadone is available in 5-mg, 10-mg, and 40-mg tablets, as well as oral solutions of 5 mg per 5 mL, 10 mg per 5 mL, and 10 mg per mL. Unlike sustained-release formulations of morphine and oxycodone, methadone tablets can be divided. Table 5 shows a comparison of estimated monthly drug costs. (31)
The authors thank Richard W. Rosenquist, M.D., and Stewart B. Leavitt, Ph.D., for assistance in the preparation of the manuscript.
The authors indicate that they do not have any conflicts of interest. Sources of funding: none reported.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army Service at large.
This article is one in a series coordinated by Allen F. Shaughnessy, Pharm.D., Tufts University Family Medicine Residency, Malden, Me.
REFERENCES
(1.) Chen KK. Pharmacology of methadone and related compounds. Ann N Y Acad Sci 1948;51:83-4.
(2.) Methadone listing as new drug with special requirements and opportunity for hearing. Federal Register 1972;37(242):26790-807. Accessed online November 10, 2004, at: http://www.nida.nih.gov/pdf/monographs/08.pdf.
(3.) Restrictions on distribution of methadone. Federal Register 1976;41(135):28261-4. Accessed online November 10, 2004, at: http:// www.nida.nih.gov/pdf/monographs/08.pdf.
(4.) Gourlay GK, Cherry DA, Cousins MJ. A comparative study of the efficacy and pharmacokinetics of oral methadone and morphine in the treatment of severe pain in patients with cancer. Pain 1986;25:297-312.
(5.) Garrido MJ, Troconiz IF. Methadone: a review of its pharmacokinetic/pharmacodynamic properties. J Pharmacol Toxicol Methods 1999;42:61-6.
(6.) Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet 2002;41:1153-93.
(7.) Mercadante S, Sapio M, Serretta R, Caligara M. Patient-controlled analgesia with oral methadone in cancer pain: preliminary report. Ann Oncol 1996;7:613-7.
(8.) Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer 2001;9:73-83.
(9.) Hewitt DJ. The use of NMDA-receptor antagonists in the treatment of chronic pain. Clin J Pain 2000;16 (2 suppl):S73-9.
(10.) Callahan RJ, Au JD, Paul M, Liu C, Yost CS. Functional inhibition by methadone of N-methyl-D-aspartate receptors expressed in Xenopus oocytes: stereospecific and subunit effects. Anesth Analg 2004;98:653-9.
(11.) De Conno F, Groff L, Brunelli C, Zecca E, Ventafridda V, Ripamonti C. Clinical experience with oral methadone administration in the treatment of pain in 196 advanced cancer patients. J Clin Oncol 1996;14:2836-42.
(12.) Gardner-Nix JS. Oral methadone for managing chronic nonmalignant pain. J Pain Symptom Manage 1996;11:321-8.
(13.) Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev 2004;(2):CD003971.
(14.) Mercadante S, Casuccio A, Fulfaro F, Groff L, Boffi R, Villari P, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol 2001;19:2898-904.
(15.) Evidence-based recommendations for medical management of chronic non-malignant pain: reference guide for physicians. The College of Physicians and Surgeons of Ontario. November 2000. Accessed online November 10, 2004, at: http://www.cpso.on.ca/Publications/pain.htm.
(16.) Morley JS, Makin MK. The use of methadone in cancer pain poorly responsive to other opioids. Pain Reviews 1998;5:51-8. Accessed online November 10, 2004, at: http://www.globalrph.com/narcoticonv.htm.
(17.) Indelicato RA, Portenoy RK. Opioid rotation in the management of refractory cancer pain. J Clin Oncol 2002;20:348-52.
(18.) American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. Glenview, Ill.: American Pain Society, 2003:16.
(19.) Ripamonti C, De Conno F, Groff L, Belzile M, Pereira J, Hanson J, et al. Equianalgesic dose/ratio between methadone and other opioid agonists in cancer pain: comparison of two clinical experiences. Ann Oncol 1998;9:79-83.
(20.) Ripamonti C, Groff L, Brunelli C, Polastri D, Stavrakis A, De Conno F. Switching from morphine to oral methadone in treating cancer pain: what is the equianalgesic dose ratio? J Clin Oncol 1998;16:3216-21.
(21.) Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage 2001;22:672-87.
(22.) Ayonrinde OT, Bridge DT. The rediscovery of methadone for cancer pain management. Med J Aust 2000;173:536-40.
(23.) Shir Y, Rosen G, Zeldin A, Davidson EM. Methadone is safe for treating hospitalized patients with severe pain. Can J Anesth 2001;48:1109-13.
(24.) Bruera E, Sweeney C. Methadone use in cancer patients with pain: a review. J Palliat Med 2002;5:127-38.
(25.) Mercadante S, Casuccio A, Agnello A, Barresi L. Methadone response in advanced cancer patients with pain followed at home. J Pain Symptom Manage 1999;18:188-92.
(26.) De Castro J, Aguirre C, Rodriguez-Sasiain JM, Gomez E, Garrido MJ, Calvo R. The effect of changes in gastric pH induced by omeprazole on the absorption and respiratory depression of methadone. Biopharm Drug Dispos 1996;17:551-63.
(27.) Bouer R, Barthe L, Philibert C, Tournaire C, Woodley J, Houin G. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: in vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol 1999;13:494-500.
(28.) Tatro DS. Drug interaction facts: the authority on drug interactions. St. Louis, Mo.: Facts and Comparisons, 2005:490-3.
(29.) Hagen NA, Wasylenko E. Methadone: outpatient titration and monitoring strategies in cancer patients. J Pain Symptom Manage 1999;18:369-75.
(30.) Ernst E, Bartu A, Popescu A, Ileutt KF, Hansson R, Plumley N. Methadone-related deaths in Western Australia 1993-99. Aust N Z J Public Health 2002;26:364-70.
(31.) 2004 Drug topics red book: the pharmacist's trusted companion for more than a century. Montvale, N.J.: Medical Economics, 2004.
JAMES D. TOOMBS, M.D., is a staff physician in the Division of Pain Medicine/Primary Care at the Harry S. Truman Memorial Veterans' Hospital in Columbia, Mo. He completed a fellowship in pain medicine at the University of Iowa Roy J. and Lucille A. Carver College of Medicine in Iowa City. A graduate of the University of Missouri-Columbia School of Medicine in Columbia, he completed a family medicine residency at the Cox Family Practice Residency Program in Springfield, Mo.
LEE A. KRAL, PHARM.D., B.C.P.S., is clinical pharmacy specialist with the Pain Medicine Division at the University of Iowa Hospitals and Clinics in Iowa City. She graduated from the University of Iowa and completed a pharmacy practice residency at the University of Iowa Hospitals and Clinics. She also serves as adjunct professor at the University of Iowa College of Pharmacy.
Address correspondence to Lee A. Kral, Pharm.D., Center for Pain Medicine and Regional Anesthesia, University of Iowa Hospitals and Clinics, 200 Hawkins Dr., Iowa City, IA 52242-1079 (e-mail: lee-kral@uiowa.edu). Reprints are not available from the authors.
COPYRIGHT 2005 American Academy of Family Physicians
COPYRIGHT 2005 Gale Group