ABBREVIATIONS: ADHD = attention-deficit hyperactivity disorder; CNS = central nervous system; MDA = 3,4-methylenedioxyamphetamine; MDMA = 3,4-methylenedioxymethamphetamine; NC = not controlled; OTC = over the counter.
INDEX TERMS: psychostimulants.
Clin Lab Sci 2005;18(2):107
The name stimulant can be given to any drug that increases the rate of a physiologic function. The term psychostimulant is more specific, referring to compounds that have direct neurological effects, typically: heightened alertness, increased energy, appetite suppression, and sometimes euphoria. Use of psychostimulants is widespread and occurs in both recreational and clinical settings. Therapeutic monitoring and screening for use and abuse of these drugs is common in clinical laboratories. Understanding the physiological effects and uses of stimulants will be of value to clinical and forensic laboratory scientists.
EXAMPLES OF PSYCHOSTIMULANTS
Table 1 lists several psychostimulants that are commonly prescribed, have widespread illicit use, or are well-known by the general public. Table 1 also identifies the control schedule of each compound. Many psychostimulants are listed as controlled substances under the Controlled Substances Act, Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970. Schedules I through IV are assigned to compounds based on their abuse potential and their medical utility. Schedules II through V contain drugs which have known medical uses whereas Schedule I compounds have no current, sanctioned medical use.
THERAPEUTIC USES OF PSYCHOSTIMULANTS
Amphetamine has long been known to be a mental stimulant. Because of its psychostimulant properties, amphetamine has been used successfully by U.S. fighter pilots because it enhances cockpit performance by reducing the effects of fatigue.1 Since amphetamine heightens alertness it has found use in the treatment of narcolepsy and in attention-deficit hyperactivity disorder.2 The drug Adderall® for example, is used widely in cases of ADHD and is merely a mixture of amphetamine s stereoisomers: dextro- and levoamphetamine.
Amphetamine also has appetite suppressant effects and thus is used in the treatment of obesity, although it is not FDA approved for this use. Although amphetamine can be considered the prototype psychostimulant, many psychostimulants have been identified or created that resemble amphetamine in their chemical structures. These compounds vary in their effects and utility as well as their abuse potential. Methamphetamine, like amphetamine has a history of illicit use and is known to be considerably more potent in vivo than the unmethylated amphetamine.3,4 Methamphetamine, like amphetamine, has been used with some success in ADHD patients. It is also approved for use in treating obesity.5
Pseudoephedrine, the name given to the 1R2R enantiomer, and ephedrine, the 1R2S enantiomer, are common medications used primarily as nasal decongestants. Unlike amphetamine and methamphetamine they have little abuse potential allowing them to be obtained OTC.6 Ephedrine is also approved for treatment of bronchospasm, enuresis, hypotension, and narcolepsy.5
Some psychostimulants have been found to have uses that are not typical for other drugs in the same class. Although bupropion is a phenylalkylamine, the structural dissimilarity with amphetamine is significant enough to produce considerably different physiologic effects. Bupropion is an example of a weak psychostimulant that has found use as an antidepressant (Wellbutrin®) and is also marketed as a smoking cessation aid (Zyban®). Although still a mild psychostimulant, the behavioral effects of bupropion are reported to be unlike amphetamine when used in humans. The abuse potential is also considerably less than with amphetamine.7 Off-label uses of bupropion include treatment of ADHD, diabetic neuropathy, and neuropathic pain.5 Another clinically-useful β-ketone psychostimulant is diethylpropion. Diethylpropioii is currently used only in the treatment of obesity. This psychostimulant is commonly prescribed as an appetite suppressant under the name Tenuate®.
Methcathinone is a Schedule I psychostimulant that is easily synthesized from ephedrine. Methcathinone is the Nmethylated version of cathinone, a natural psychostimulant obtained from the Catha edulis plant and known as "qat" or "khat".8 Methcathinone has no sanctioned medical use and is only used recreationally. Its effects are those of a classic psychostimulant, causing 'flying euphoria followed by a five-to-eight hour period of feeling tough, invincible with increased libido, and desire to be physical.8,9
The next two compounds listed in Table 1 are also Schedule I compounds. 3,4-methylenedioxyamphetamine (MDA) and 3,4-methylenedioxymethamphetamine (MDMA) are popular recreational drugs known respectively as 'the love (or hug) drug' and 'ecstasy'. MDMA use is growing rapidly and in some European countries is the second most frequently used illegal drug after marijuana.10 Interestingly, both MDMA and MDA were used therapeutically before being classified Schedule I in 1985. These compounds were used by patients in constructive psychotherapy sessions.8 MDMA has received much negative attention in recent years as claims of its neurotoxicity mounted. However data concerning the neurotoxicity of MDMA have been inconsistent and one major study was even retracted after it was found that the toxicity in the study was brought about by methamphetamine rather than MDMA.11,12 The widespread use and popularity of MDMA coupled with the lack of definitive reports on neurotoxicity have encouraged some to reconsider the utility of this drug.13-15 Because of MDMAs unique effects, a new study investigating the therapeutic use of MDMA has recently earned IRB and FDA approval. This research will assess the use of MDMA in the treatment of post-traumatic stress disorder.16,17
Perhaps the most well-known psychostimulant after amphetamine is methylphenidate, made popular under the trade name Ritalin®. Methylphenidate has pharmacological effects similar to amphetamine; however, its abuse potential is somewhat lower, although there are many conflicting reports regarding methylphenidate abuse potential (see reference 18 for review). Methylphenidate has become the drug of choice for treating ADHD but has also found offlabel use as an antidepressant.5
Two other drugs worth mentioning as psychostimulants are phentermine and fenfluramine. Both of these drugs have been used as appetite suppressants and both have amphetamine-like sympathomimetic effects. Fenfluramine contains a chiral center and fenfluramine is the name given to the racemic mixture of D- and L-isomers. The D-isomer (dexfenfluramine) was purportedly responsible for the anorectic actions of fenfluramine and was also associated with fewer side effects than the racemic fenfluramine. Dexfenfluramine was marketed as Redux®.19 By 1997 both fenfluramine and dexfenfluramine were removed from the U.S. market at the request of the FDA when cases of cardiac valvulopathy were reported.5,20,21 Fenfluramine is perhaps best known by its off-label use with phentermine, known as "Fen-phen". Fen-phen was widely used for the long-term management of obesity. The fenfluramine-phentermine combination was also associated with valvulopathies and a bulletin was issued by the FDA to cease off-label use of the Fen-Phen combination.5,21 Of the two drugs, only phentermine is still used as an appetite suppressant although tolerance to the anorexiant effects of phentermine usually develops a few weeks after starting therapy.
When considering the clinically-used drugs listed in Table 1, cocaine is the only one that is not used for its psychostimulant effects. Cocaine is used clinically only as a local anesthetic, usually in mucosal or ophthalmic procedures.
MECHANISM OF ACTION
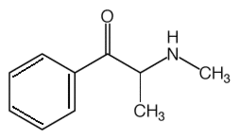
It is easy to see the resemblance between the chemical structures of common psychostimulants and endogenous monoamine neurotransmitters (Figure 1). The prototype psychostimulant amphetamine closely resembles the catecholamine neurotransmitters norepinephrine, epinephrine, and dopamine. Since many psychostimulants share the features of a phenyl ring, a nitrogen group, and carbon side chains of varying lengths, many stimulants fall into the category of phenylalkylamines. With the possible exception of cocaine, all the compounds listed in Table 1 can be classified as phenylalkylamines. Because amphetamine is considered the prototype stimulant, other compounds that have similar chemical structures and similar physiologic effects are often termed 'amphetamines' (Figure 2).
Given their structural similarity to endogenous neurotransmitters, it is not surprising that many phenylalkylamines have autonomie nervous system activity, i.e., sympathomimetics, as well as mood-altering effects. Amphetamine and other closely related phenylalkylamines can activate receptors that normally bind catecholamines or serotonin. In addition, amphetamine and related compounds can cause the release of catecholamines and serotonin from nerve endings.22-26 Once released, the endogenous neurotransmitters are free to act on their extracellular receptors. When catecholamine receptors in the brain are activated, myriad effects can result. Neuropsychological effects of catecholamine receptor activation or potentiation by psychostimulants can include: increased alertness, insomnia, euphoria, decreased appetite, and at higher doses, psychosis. Indeed, amphetamine can correctly be called a "psychotomimetic" since high doses can bring about a psychosis very similar to that seen in schizophrenic patients.27,28 In the periphery, activation or potentiation of catecholamine receptors by psychostimulants can result in: vasoconstriction and subsequent hypertension, mydriasis, tachycardia, and other general sympathomimetic effects. The mechanism of action of amphetamine and amphetamine-like psychostimulants involves four major effects:
1. Binding to extracellular catecholamine receptors
2. Inhibition of monoamine neurotransmitter uptake
3. Release of catecholamines from neurons
4. Inhibition of monoamine oxidase
Psychostimulants alter neurotransmitter release. Psychostimulants can effectively alter the amount or the rate of neurotransmitter released by a monoaminergic neuron. The resulting change in mood or behavior is the summation of these neuromodulations and is quite complex. At the cellular level this neuromodulation is due to one or more of the effects listed above. Amphetamine-like compounds have a wide range of affinities to catecholamine and serotonin receptors. The overall pattern of receptor binding for a psychostimulant is unique for a given stimulant and contributes to the distinctive behavioral effects of a given compound.
In addition to having direct receptor-binding effects, many psychostimulants inhibit monoamine transporters. Monoamine transporter proteins serve to recycle neurotransmitters after they are released from the neuron and in so doing, terminate the neurotransmitter signal. These same monoamine transporters are also the targets for antidepressant medications including the popular drugs fluoxetine (Prozac®), paroxetine (Paxil®) and sertraline (Zoloft®). Inhibitors of monoamine transporters block the uptake, or 'reuptake', of neurotransmitters by neurons (Figure 3). This blockade effectively increases the concentration of neurotransmitter in the synapse, resulting in increased binding of neurotransmitters to their receptors.
Psychostimulants can also elevate the concentration of neurotransmitter in the synapse by evoking release of neurotransmitters. The release of stored neurotransmitters can be triggered directly when psychostimulants bind receptors present on neurons or can release neurotransmitters indirectly via an exchange mechanism occurring through monoamine transporter proteins. The exchange mechanism or efflux process which can occur via monoamine transporters has been observed with many amphetamine derivatives.29,30 This efflux is thought to be a type of 'reverse-transport' mediated by monoamine transporters.31,32 Finally, amphetamine and amphetamine-like psychostimulants often act as competitive inhibitors of the enzyme monoamine oxidase (MAO).33,34 MAO is a mitochondrial enzyme that breaks down monoamine neurotransmitters. Products of catecholamine breakdown include 3-methoxy-4-hydroxymandelic acid (VMA), homovanillic acid (HVA), and dihydroxyphenylacetic acid (DOPAC). These compounds are commonly-measured metabolites of catecholamines whose formation is due in part or in full to MAO. Inhibition of MAO would thus bring about an expected increase in monoamine neurotransmitters since their breakdown would be impeded.
In summary, psychostimulants can affect neurotransmitter release in at least four ways; by binding extracellular receptors, by blocking monoamine transporters, by evoking neurotransmitter release, and by antagonizing the enzyme MAO (Figure 3). A given psychostimulant can have multiple mechanisms of actions. Indeed, the actions of some psychostimulants, such as MDMA and bupropion have not yet been fully explained but it is clear that all or some of the mechanisms discussed play a role in the unique actions of these drugs.
PHYSIOLOGIC EFFECTS OF PSYCHOSTIMULANTS
Having already considered the cellular actions of psychostimulants it is worth mentioning the overall physiologic effects of these drugs. Because of the complex circuitry in the brain and the overlap of monoamine neurotransmitter systems, understanding how subtle differences in chemical structure bring about significantly different psychological effects can be difficult. Although all differ in their overall physiological effects, the psychostimulants listed in Table 1 do share the following adverse side effects: agitation/anxiety, dizziness, restlessness, psychosis, and seizures. Many of these compounds can also cause hypertension, insomnia, hyperthermia, tachycardia, and euphoria.5 A discussion of the psychopharmacological mechanisms of action of each individual psychostimulant is beyond the scope of this article (see reference 35 for excellent reviews).
In general, the physiologic effects of psychostimulants can be represented by the prototype psychostimulant, amphetamine. In the periphery amphetamine acts as a sympathomimetic. Mydriasis, hypertension, and tachycardia would be expected from a compound with close structural similarity to endogenous catecholamines. Amphetamine releases norepinephrine from sympathetic nerve endings which causes increases in systolic and diastolic blood pressure, often causing a reflexdecrease in heart rate. At higher doses or overdoses tachycardia or cardiac arrhythmias can occur.5 Because of these cardiovascular effects, amphetamine is contraindicated in patients with arteriosclerosis, coronary artery disease, hypertension, and closed-angle glaucoma.5 Interestingly, the commonly used drugs ephedrine and pseudoephedrine are utilized for their vascular effects. By acting directly on alpha-adrenergic receptors in the mucosa of the respiratory tract, pseudoephedrine produces vasoconstriction leading to nasal decongestion.
In the CNS, amphetamine modulates monoamine neurotransmitter release and kinetics. The overall effect of these actions is consistent with known roles of monoamines in the brain. The anorectic effects of amphetamine involve release of norepinephrine in the paraventricular nucleus of the hypothalamus, a brain area central to feeding behavior.36,37 Serotonergic systems also play a role in appetite and satiety and these systems are also affected by psychostimulants like amphetamine.38,39 In addition to appetite control, amphetamine is also used for its ability to focus attention. The basis for this effect is not well understood. The seemingly paradoxical fact that psychostimulants can act as calming agents in humans seems enigmatic. The effects of amphetamine on attention and alertness are ultimately due to the modulation of normal patterns of central activity. These modulations are brought about by modulating serotonergic, dopaminergic, and noradrenergic pathways but the precise mechanism of action of amphetamine in ADHD is unknown (see references 2 and 40 for reviews).
Euphoria, of course, is an important, high-dose effect of amphetamine. The euphoric effects of amphetamine and methamphetamine are very similar to cocaine however unlike cocaine, amphetamine and methamphetamine are taken orally and are metabolized more slowly than cocaine, making it the psychostimulant of choice for many drug users. Centrally, amphetamine and cocaine cause an acute dopamine release in addition to inhibiting neuron dopamine uptake.41'43 This release of dopamine in the nucleus accumbens and prefrontal cortex is thought to cause the prominent euphoric and reinforcing effects associated with amphetamine and cocaine.44 These psychostimulants can produce a significant euphoria, locomotor stimulation, reduced fatigue, sexual stimulation, increased mental attention, and increased sociality. Higher doses can produce tremors and vomiting, as well as tonic-clonic convulsions.
CONCLUSION
Psychostimulants are important pharmacological agents used in a variety of conditions including the treatment of obesity, ADHD, narcolepsy, and as decongestants. Psychostimulants typically contain a phenylalkylamine structure that closely resembles that of endogenous monoamines. The actions of psychostimulants are mediated through their ability to bind monoamine receptors and/or modulate monoamine transmitter release. Although psychostimulants have a history of abuse and misuse their therapeutic uses are numerous and significant.
REFERENCES
1. Emonson DL, Vanderbeek RD. The use of amphetamines in U.S. Air Force tactical operations during Desert Shield and Storm. Aviat Space Environ Med 1995;66:260-3.
2. Gainetdinov RR, Wetsel WC, Jones SR, and others. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 1999:283:397-401.
3. Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav 1987:26:547-51.
4. Glennon RA. Discriminative stimulus properties of phenylisopropylamine derivatives. Drug Alcohol Depend 1986;17:119-34.
5. Clinical Pharmacology 2000 Database. 2004. Gold Standard Medical Media, http://cpip.gsm.com/ Accessed January 7, 2005.
6. Wee S, Ordway GA, Woolverton WL. Reinforcing effect of pseudoephedrine isomers and the mechanism of action. Eur J Pharmacol 2004;493:117-25.
7. Griffith JD, Carranza J, Griffith C, Miller LL. Bupropion: clinical assay for amphetamine-like abuse potential. J Clin Psychiatry 1983;44:206-8.
8. Perrine DM. The chemistry of mind-altering drugs. Washington DC: American Chemical Society; 1996.
9. EmersonTS, CisekJE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med 1993;22:1897-903.
10. Landry MJ. MDMA: a review of epidemiologic data. J Psychoactive Drugs 2002:34:163-9.
11. Ricaurte GA, Yuan J, Hatzidimitriou G, and others. Severe dopaminergic neurotoxicity in primates after a common recreational dose regimen of MDMA ('ecstasy'). Science 2002:297:2260-3.
12. Ricaurte GA, Yuan J, Hatzidimitriou G, and others. Retraction. Science 2003:301:1479.
13. Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev 2003;42:155-68.
14. Kish SJ. How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol Biochem Behav 2002:71:845-55.
15. Pentney AR. An exploration of the history and controversies surrounding MDMA and MDA. J Psychoactive Drugs 2001:33:213-21. :
16. Michael C. Mithoefer MD, Mark T, Wagner PD. Experimental proposal: a human phase II study-safety and efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy in the treatment of chronic posttraumatic stress disorder (PTSD). Charleston SC: Medical University of South Carolina, 2002.
17. Weiss R. DEA approves trial use of ecstasy in trauma cases. The Washington Post March 2, 2004:02.
18. Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential ' of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav 2001;68:611-27.
19. Bremer JM, Scott RS, Lintott CJ. Dexfenfluramine reduces cardiovascular risk factors. Int J Obes Relat Metab Disord 1994:18:199-205.
20. Shively BK, Roldan CA, Gill EA, and others. Prevalence and determinants of valvulopathy in patients treated with dexfenfluramine. Circ 1999:100:2161-7.
21. Connolly HM, Crary JL, McGoon MD, and others. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 1997:337:581-8.
22. Rubin RP, Jaanus SD. A study of the release of catecholamines from the adrenal medulla by indirectly acting sympathomimetic amines. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol 1966;254:125-37.
23. Magyar K, Knoll J. Para-substituted amphetamines and brain serotonin. Pol J Pharmacol Pharm 1975;27:139-43.
24. Paton DM. Structure-activity relations for the acceleration of efflux of noradrenaline from adrenergic nerves in rabbit atria by sympathomimetic amines. Can J Physiol Pharmacol 1975:53:822-9.
25. Carr LA, Moore KEL Release of norepinephrine and normetanephrine from cat brain by central nervous system stimulants. Biochem Pharmacol 1970;19:2671-5.
26. Fitzgerald JL, Reid JJ. Effects of methylenedioxymethamphetamine on the release of monoamines from rat brain slices. Eur J Pharmacol 1990;191:217-20.
27. Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. AmJ Psychiatry 1976;133:197-202.
28. Angrist B, Sathananthan G, WiIk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res 1974;ll:13-23.
29. Rudnick G, Wall SC. p-Chloroamphetamine induces serotonin release through serotonin transporters. Biochem 1992;31:6710-8.
30. Will SG, Gu H, Rudnick G. Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. MoI Pharmacol 1995:47:544-50.
31. Sasa M. Function of monoamine neurotransmitter transporters. Nippon Rinsho 2001:59:1457-64.
32. Scholze P, Norregaard L, Singer EA, and others. "I he role of zinc ions in reverse transport mediated by monoamine transporters. J Biol Chem 2002:277:21505-13.
33. Weyler W, Salach JI. Purification and properties of mitochondrial monoamine oxidase type A from human placenta. J Biol Chem 1985:260:13199-207.
34. Pearce LB, Roth JA. Human brain monoamine oxidase type B: mechanism of deamination as probed by steady-state methods. Biochem 1985:24:1821-6.
35. Neuropsychopharmacology, The fifth generation of progress. Philadelphia: Lippincott, Williams and Wilkins, 2002.
36. Prankish HM, Dryden S, Hopkins D, and others. Neuropeptide Y, the hypothalamus, and diabetes: insights into the central control of metabolism. Peptides 1995:16:757-71.
37. Samanin R, Garattini S. Neurochemical mechanism of action of anorectic drugs. Pharmacol Toxicol 1993:73:63-8.
38. Blundell JE, Leshem MB. The effect of 5-hydroxytryptophan on food intake and on the anorexic action of amphetamine and fenfluramine. J Pharm Pharmacol 1975:27:31-7.
39. Halford JC, Blundell JE. Separate systems for serotonin and leptin in appetite control. Ann Med 2000:32:222-32.
40. Ohno M. The dopaminergic system in attention deficit/hyperactivity disorder. Congenit Anom (Kyoto) 2003:43:114-22.
41. Karoum F, Chrapusta SJ, Brinjak R, and others. Regional effects of amphetamine, cocaine, nomifensine, and GBR 12909 on the dynamics of dopamine release and metabolism in the rat brain. BrJ Pharmacol 1994;! 13:1391-9.
42. Reid MS, Hsu K, Jr., Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse 1997:27:95-105.
43. Jacocks HM 3rd, Cox BM. Serotonin-stimulated release of [3H]dopamine via reversal of the dopamine transporter in rat striatum and nucleus accumbens: a comparison with release elicited by potassium, N-methyl-D-aspartic acid, glutamic acid and D-amphetamine. J Pharmacol Exp Ther 1992;262:356-64.
44. Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse 1989:4:156-61.
Kevin F Foley PhD MT(ASCP) is with the Department of Biomedical Technologies, University of Vermont, Burlington VT.
Address for correspondence: Kevin F Foley PhD MT (ASCP), Assistant Professor, University of Vermont, Department of Medical Laboratory and Radiation Sciences, 302 Rowell Building, 106 Carrigan Drive, Burlington VT 05405. (802) 656-2506. kfoley@uvm.edu.
Victor A Skrinska PhD DABCC is the Focus: Psychostimulants guest editor.
Focus Continuing Education Credits: see pages 124 to 126 for learning objectives, test questions, and application form.
Copyright American Society for Clinical Laboratory Science Spring 2005
Provided by ProQuest Information and Learning Company. All rights Reserved