Rationale: High-altitude pulmonary edema (HAPE) is characterized by excessive pulmonary vasoconstriction and is associated with decreased concentrations of nitric oxide (NO) in the lung. Objectives: We hypothesized that individuals susceptible to HAPE (HAPE-S) would also have dysfunction of the vascular NO vasodilator pathway during hypoxia in the systemic vasculature. Methods: During normoxia (FI^sub O^sub 2^^ = 0.21) and 4 hours of normobaric hypoxia (FI^sub O^sub 2^^ = 0.12, corresponding to an altitude of 4,500 m above sea level) endothelium-dependent and endothelium-independent vasodilator responses to intraarterial infusion of acetylcholine (ACh) and sodium nitroprusside, respectively, were measured by forearm venous occlusion plethysmography in nine HAPE-S subjects and in nine HAPE-resistant control subjects. Main Results: Pulmonary artery systolic pressure increased from 22 ± 3 to 33 ± 6 mm Hg (p
Keywords: edema; endothelin; endothelium; hypoxia; nitric oxide
High-altitude pulmonary edema (HAPE) is a life-threatening form of noncardiogenic pulmonary edema that is characterized by pulmonary hypertension and elevated capillary pressure in the pulmonary circulation (1, 2). The increase in intravascular hydrostatic pressure augments fluid filtration across the alveolar capillary barrier into the alveolar space and is believed to be a key factor in the pathogenesis of HAPE (3). Individuals susceptible to HAPE (HAPE-S) have both an augmented pulmonary vasoconstrictor response to hypoxia (4) and an enhanced increase of pulmonary artery systolic pressure (PASP) during exercise in normoxia (5). The underlying mechanisms predisposing to exaggerated pulmonary vasoconstriction in HAPE-S individuals are not known, but several studies suggest that susceptibility to HAPE may be associated with reduced pulmonary synthesis of the endothelium-derived vasodilator nitric oxide (NO) and an impairment of NO-mediated pulmonary vasodilation. Indeed, constitutively released NO mediates relaxation of the pulmonary vascular bed in healthy individuals both during normoxia and acute hypoxia (6). Moreover, exhaled NO is decreased in HAPE-S individuals during exposure to normobaric hypoxia (7) and also in patients with established HAPE (8). In both studies, a negative correlation between exhaled NO and the extent of hypoxic pulmonary vascular responses was shown. In another study, decreased concentrations of the NO metabolites nitrite and nitrate were observed in bronchoalveolar lavage fluid in HAPE-S subjects (9), supporting the hypothesis of an impairment of NO formation or metabolism. Although these findings all point to a possible impairment of NO production by the lung vascular endothelium during hypoxia, they offer no direct proofof-concept because measurements of exhaled NO and alveolar fluid NO metabolites only imperfectly reflect pulmonary vascular NO production (10, 11).
We hypothesized that susceptibility to HAPE is related to a generalized hypoxia-induced vascular endothelial dysfunction with subsequent reduction of NO bioavailability. Therefore, we directly assessed by blood flow measurements the effect of hypoxia on the function of the systemic vascular endothelium of HAPE-S and HAPE-resistant control subjects in a single-blind, randomized trial.
Some of the results of these studies have been previously reported in the form of an abstract (12).
METHODS
Study Population
We enrolled 18 healthy mountaineers, whose susceptibility or resistance to HAPE was known from their participation in previous studies at high altitude (3, 9, 13). All participants were nonsmokers living at low altitude. Volunteers with hyperhomocysteinemia, diabetes, or cardiac or pulmonary diseases, including airway infections, were excluded from the study. In terms of other known conditions associated with endothelial dysfunction (hyperlipidemia, arterial hypertension), the groups were matched (Table 1). In each group, there was one subject with mild hypertension, treated with 25 mg metoprolol daily.
The study was conducted in accordance with the Declaration of Helsinki and its current amendments, and was approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg. Before the study, all participants provided written, informed consent.
General Procedures
The study was performed as a single-blind, randomized trial, in which the participants were blinded with respect to the FI^sub O^sub 2^^, and the investigators were blinded with respect to the participants' history of HAPE. The participants were studied in a normobaric hypoxia room that provided a constant FI^sub O^sub 2^^ level of 0.21 (normoxia) or 0.12 (hypoxia) by admixture of N^sub 2^-enriched air. Each participant was exposed to 4 hours of normoxia and 4 hours of normobaric hypoxia in randomized order while comfortably resting in a supine position. After an equilibration period of 90 minutes, blood samples were taken for determination of plasma nitrite and plasma endothelin 1 (ET-1). Thereafter, endothelium-dependent and endothelium-independent vasodilator responses and PASP were assessed. Afterwards, the Fi^sub O2^ was switched accordingly to 0.21 or 0.12. After another equilibration period of 90 minutes, all measurements were repealed in the same order as in the morning session.
In the preceding 12 hours and during the study, the participants were not allowed to perform any physical exercise or consume methylxanthine-containing food and beverages. Fruits and salads were also not provided to minimize dietary nitrite intake.
Assessment of Endothelium-dependent and Endothelium-independent Vasodilator Responses
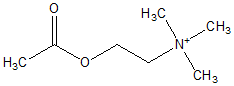
To measure drug-induced changes of forearm blood (low (FBF), venous occlusion plethysmography with strain gauges placed around the forearms was performed as described previously (14,15). FBF was measured by the rate of increase in forearm volume during periods of venous occlusion achieved by rapid inflation of an upper arm cuff to 40 mm Hg. During FBF measurements, the hand was excluded from the circulation by inflating a wrist cuff to suprasystolic pressures. Changes in vascular reactivity were assessed observing flow responses to different doses of vasoactive agents. Under local anesthesia, a catheter was inserted into the brachial artery. After resting FBF levels had been established, the following compounds were administered intraarterially: (1) sodium nitroprusside (SNP), to assess endothelium-independent vasodilation, and (2) acetylcholine (ACh), to stimulate endothelium-dependent vasodilation. Both substances were infused in randomized order in six increasing dose rates (SNP: 0.006 - 0.03 - 0.075 - 0.15 0.3 - 0.6 µg/minute/100 ml forearm volume; ACh: 0.01 - 0.05 - 0.2 1 - 4 - 16 µg/minute/100 ml forearm volume) for a period of 5 minutes each, with SNP and ACh infusions separated by a wash-out phase of at least 30 minutes. Area under the dose-response curves was calculated from each individual's dose-response curve plotted in linear scaling. Arterial blood pressure, ECG, and oxygen saturation were continuously monitored.
Echocardiography
PASP was estimated from peak tricuspid regurgitation jet velocities according to the following equation: PASP = 4V^sup 2^ + 5 mm Hg, with V being the peak velocity (in m/second) of tricuspid regurgitation jet, and 5 mm Hg being the estimated right atrial pressure (16).
Measurement of Plasma Nitrite, Nitrate, and ET-1
Plasma nitrite concentrations were determined by a flow injection analysis technique based on the Griess reaction (17). Nitrate was determined after reduction to nitrite by preincubation with vanadium chloride (18) and subsequent detection by the flow injection analysis system. Plasma ET-1 was measured by radioimmunoassay as described previously (19).
Statistics
Local hemodynamic responses to infusion of study drugs (normoxia vs. hypoxia) were compared by using two-way repeated measures analysis of variance. Two-tailed paired and unpaired t tests were used for comparisons within and between the groups, respectively. Pearson correlation was performed to analyze the influence of selected factors on vasodilator responses, and p values less than 0.05 were considered statistically significant. Unless otherwise indicated, data are expressed as mean values ± SD.
RESULTS
Baseline clinical characteristics of the study population were similar between HAPE-S and control subjects (Table 1), and in each group, one participant with hypertension was treated with metoprolol.
Effects of Hypoxia on Gas Exchange, Systemic Hemodynamics, and PASP
There were no differences between HAPE-S and control subjects in any of the baseline parameters during normoxia, and the changes in oxygen saturation, heart rate, and mean arterial blood pressure induced by hypoxia were similar in both groups (Table 2). In all participants, the decrease of oxygen saturation was accompanied by an increase in PASP, which was substantially higher in HAPE-S than in control subjects.
Baseline FBF and Vasodilator Response to ACh and SNP
Baseline FBF was similar during normoxia in both groups. Hypoxia induced an increase in FBF in control subjects but not in HAPE-S subjects (Table 2).
Neither ACh nor SNP exerted systemic vasorelaxant effects, as judged by the absence of changes in blood pressure, heart rate, and FBF in the control arm. The dose-response relationship of intraarterially administered ACh for HAPE-S and control participants is shown in Figure 1A. In HAPE-S subjects, hypoxia impaired ACh-dependent increase in FBF, whereas in control participants, endothelium-dependent relaxation was similar in normoxia and hypoxia. During hypoxia, there was a negative correlation between PASP and the area under the dose-response curve of ACh-induced FBF (Figure 2). However, SNP-induced relaxation slightly increased in control subjects during hypoxia, whereas there was no change in HAPE-S subjects (p = 0.21; Figure 1B).
Plasma Concentrations of Nitrite, Nitrate, and ET-1
Hypoxia induced a marked decrease of plasma nitrite in HAPE-S (p = 0.004) but not in control subjects, whereas plasma nitrate remained unaffected in both groups (Table 2). In HAPE-S subjects, during hypoxic exposure, a positive correlation existed between plasma nitrite and the area under the dose-response curve of ACh-induced FBF (Figure 3). Plasma ET-1 increased during hypoxia in both groups without a difference between HAPE-S and control subjects (Table 2).
DISCUSSION
To the best of our knowledge, this is the first study to directly measure the function of the vascular endothelium in HAPE-S individuals. The primary finding of the study is that, in individuals with previously well documented HAPE, but not in control participants, hypoxia reduced the endothelium-dependent vasodilator responsiveness of forearm resistance vessels to intraarterial infusion of ACh, a gold-standard test to assess endothelial function. The shift of the dose-response curve to ACh induced by hypoxia was comparable to that induced by cardiovascular risk factors such as hypertension (20) or smoking (21). Endothelium-independent vasodilation to SNP was unchanged, indicating that the decreased response to ACh was caused by a reduction in endothelial function rather than an attenuation of vascular smooth muscle function. Because ACh responses in the human forearm are almost exclusively mediated by NO release through activation of endothelial NO synthase (NOSIII) (22), and only to a small extent through prostaglandins (23), our findings strongly suggest that hypoxia induces endothelial dysfunction in HAPE-S individuals, resulting in decreased vasodilator responsiveness in the systemic circulation.
The potential impairment of the NO pathway in HAPE-S individuals is further supported by the finding that plasma concentrations of nitrite, an oxidative metabolite of NO, decreased in HAPE-S subjects during hypoxic exposure, and that this decrease correlated with the ACh responsiveness of the arterial bed. Because changes in plasma nitrite concentrations in the human forearm circulation accurately reflect acute changes in the activity of NOSIII (22), a constitutive enzyme that catalyzes endothelial NO synthesis from L-arginine (24), the reduction of plasma nitrite is a marker of decreased NO bioavailability (25).
In contrast to nitrite, plasma nitrate concentrations did not change during hypoxic exposure in HAPE-S or in control subjects, which is not surprising because only a small fraction is derived from endogenous NO and nitrate is influenced by a variety of NOSIII-independent factors (25). Moreover, the nitrate half-life of 5 to 8 hours in humans is considerably longer than that of nitrite and therefore acute changes in NOSIII activity will not readily be mirrored in its plasma concentration, further limiting its usefulness in short-term intervention studies (25).
The selective reduction of endothelial NO bioavailability in the systemic vasculature only observed in HAPE-S individuals during hypoxia fits well with recent studies demonstrating reduced NO bioavailability in the pathophysiology of HAPE: concentrations of nitrite and nitrate in bronchoalveolar lavage fluid are decreased in HAPE-S individuals after 2 days at 4,559 m, but not in control subjects (9). Congruently, the enhanced hypoxic pulmonary vascular response in HAPE-S individuals is associated with less NO in exhaled air (7, 8), and conversely, the inhalation of NO profoundly lowers PASP in HAPE-S individuals while having only mild effects in control subjects (13). Furthermore, phosphodiesterase-5 inhibitors, which slow down the degradation of cyclic guanosine monophosphate (cGMP), reduce hypoxic pulmonary vasoconstriction (26), improve performance (27, 28), and prevent HAPE (29). Although the systemic and the pulmonary circulation are regulated differently in hypoxia, it is conceivable that similar mechanisms account for the systemic endothelial dysfunction in hypoxia and contribute to the decreased bioavailability of NO in the lung of HAPE-S subjects, because we found an inverse correlation between PASP and the respective vasodilator response to ACh in the systemic circulation.
The reasons for the reduced NO bioavailability in HAPE-S subjects are unclear and may include reduced NOS substrate and cofactor availability, increased concentrations of endogenous NOS-inhibiting L-arginine analogs, differences in endothelial cell membrane proteins that alter NOS activity, downregulation of NOS, or increased NO breakdown. However, this study was not designed to answer the question of which of these parameters are primarily affected by hypoxia and responsible for the impairment of NO homeostasis in HAPE-S subjects. But the preserved vasodilator responsiveness to SNP suggests that vascular smooth muscle responses in HAPE-S individuals are well retained and that NO action itself is not critically impaired.
Hypoxic exposure was associated with increases in plasma ET-1, which is in line with previous studies (30). However, our finding of an impaired NO pathway may explain why a similar hypoxia-induced increase of plasma ET-1 is associated with a greater increase in PASP in HAPE-S subjects. ET-1 binds to two types of receptors: ET^sub A^ and ET^sub B^. Stimulation of ET^sub A^ and ET^sub B^ receptors on smooth muscle causes vasoconstriction, whereas stimulation of endothelial ET^sub B^ receptors causes vasodilation through release of NO and prostaglandins (31). ET^sub A^ receptor-mediated vasoconstriction contributes to basal systemic and pulmonary vascular tone; however, acute hypoxic pulmonary vasoconstriction is not primarily mediated by stimulation of ET^sub A^ receptors, as supported by the fact that the ET^sub A^ receptor antagonist BQ-123 did not reduce pulmonary vascular tone in healthy individuals during acute hypoxia (32). In contrast, ET^sub B^ receptor-mediated vasodilation may play a major physiologic role in modulating vascular tone in reaction to acute hypoxia. In an animal model, hypoxia suppresses ET^sub B^ receptor-mediated stimulation of NO synthesis in hypoxia-induced hypertensive rat lungs and thus contributes to the development of pulmonary hypertension at least partly (33). Moreover, transgenic rats with ET^sub B^ receptor deficiency develop exaggerated pulmonary vasoconstriction when exposed to acute hypoxia (34). Thus, ET^sub B^ receptor-induced NO-mediated vasodilation may be impaired in HAPE-S individuals in hypoxia as part of a generalized state of vascular endothelial dysfunction and thus contributes to the exaggerated pulmonary vasoconstriction.
In conclusion, we have shown that susceptibility to HAPE is associated with hypoxia-induced endothelial dysfunction, resulting in impaired endothelium-dependent vasodilation in the systemic circulation. Because endothelium-derived NO plays a central role in pulmonary vascular regulation, reduced NO bioavailability could contribute to the enhanced hypoxic pulmonary vasoconstriction that is a causal element in the pathophysiology of HAPE. If indeed a relative lack of bioavailable NO is the key factor in the pathogenesis of HAPE, then measures increasing cGMP-induced vasodilation would be expected to favorably modulate the occurrence of HAPE. Indeed, it has recently been shown, that the phosphodiesterase-5 inhibitor tadalafil, which increases cGMP levels in lung tissue by inhibition of its degradation, lowers PASP, and prevents HAPE (29).
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Acknowledgment: The authors thank all the mountaineers who took part in the study, Martina Haselmayr and Elmar Menold for their excellent technical assistance, Dr. Sidney Shaw for analyzing plasma ET-1, Putrika Gharini for analyzing plasma nitrite and nitrate, and Dr. Matthias Kohlerfor his kind suggestions planning the study.
References
1. Maggiorini M, Melot C, Pierre S, Pfeiffer F, Greve I, Sartori C, Lepori M, Hauser M, Scherrer U, Naeije R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 2001;103:2078-2083.
2. Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am J Respir Crit Care Med 2005;171:83-87.
3. Bärtsch P, Maggiorini M, Ritter M, Noli C, Vock P, Oelz O. Prevention of high-altitude pulmonary edema by nifedipine. N Engl J Med 1991; 325:1284-1289.
4. Bärtsch P. High altitude pulmonary edema. Respiration (Herrlisheim) 1997;64:435-443.
5. Grünig E, Mereles D, Hildebrandl W, Swenson ER, Kübler W, Kücherer H, Bärtsch P. Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J Am Coll Cardiol 2000;35:980-987.
6. Blitzer ML, Loh E, Roddy MA, Stamler JS, Creager MA. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol 1996;28: 591-596.
7. Busch T, Bärtsch P, Pappert D, Grünig E, Hildebrandl W, Elser H, Falke KJ, Swenson ER. Hypoxia decreases exhaled nitric oxide in mountaineers susceptible to high-altitude pulmonary edema. Am J Respir Crit Care Med 2001;163:368-373.
8. Duplain H, Sartori C, Lepori M, Egli M, Allemann Y, Nicod P, Scherrer U. Exhaled nitric oxide in high-altitude pulmonary edema: role in the regulation of pulmonary vascular tone and evidence for a role against inflammation. Am J Respir Crit Care Med 2000;162:221-224.
9. Swenson ER, Maggiorini M, Mongovin S, Gibbs JS, Greve I, Mairbäurl H, Bärtsch P. Pathogenesis of high-altitude pulmonary edema: inflammation is not an etiologic factor. JAMA 2002;287:2228-2235.
10. Berg JT, Deem S, Kcrr ME, Swenson ER. Hemoglobin and red blood cells alter the response of expired nitric oxide to mechanical forces. Am J Physiol Heart Circ Physiol 2000;279:H2947-H2953.
11. Vaughan DJ, Brogan TV, Kerr ME, Deem S, Luchtel DL, Swenson ER. Contributions of nitric oxide synthase isozymes to exhaled nitric oxide and hypoxic pulmonary vasoconstriction in rabbit lungs. Am J Physiol Lung Cell Mol Physiol 2003;284:L834-L843.
12. Berger MM, Hesse C, Dehnert C, Siedler H, Kleinbongard P, Gharini P, Bardenheuer HJ, Kelm M, Bärtsch P, Haefeli WE. Hypoxia impairs endothelial function in individuals susceptible to high-altitude pulmonary oedema (HAPE): the missing link in the pathogencsis of HAPE [abstract]? High Alt Med Biol 2004;5:476.
13. Scherrer U, Vollenweider L, Delabays A, Savcic M, Eichenberger U, Kleger GR, Fikrle A, Ballmer PE, Nicod P, Bärtsch P. Inhaled nitric oxide for high-altitude pulmonary edema. N Engl J Med 1996;334:624-629.
14. Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension 1995;25:918-923.
15. Haefeli WE, Linder L, Läscher TF. Quinaprilat induces arterial vasodilation mediated by nitric oxide in humans. Hypertension 1997;30:912-917.
16. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 1984;70:657-662.
17. Kleinbongard P, Rassaf T, Dejam A, Kerber S, Kelm M. Griess method for nitrite measurement of aqueous and protein-containing samples. Methods Enzymol 2002;359:158-168.
18. Gharini PDA, Lauer T, Matern S, Kelm M, Kleinbongard P. A simple method to measure nitrite and nitrate in urine measurement of the end product of NO-metabolisms pathway in urine through vanadium chloride and Griess. Marburg, Germany: AOL Verlag Marburg; 2002.
19. Shaw SG, Schmid M, Casty A. Critical factors in the radioimmunoassay of endothelin-1, endothelin-3, and big endothelin-1 in human plasma. Anal Biochem 2000;278:143-149.
20. Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990;323:22-27.
21. Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 1996;94:6-9.
22. Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthasc activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 2001;98:12814-12819.
23. Kamper AM, Paul LC, Blauw GJ. Prostaglandins are involved in acetylcholine- and 5-hydroxytryptamine-induced, nitric oxide-mediated vasodilatation in human forearm. J Cardiovasc Pharmacol 2002; 40:922-929.
24. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991;43:109-142.
25. Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta 1999;1411:273-289.
26. Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic peptide activity. Circulation 2003;107: 234-237.
27. Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, Seeger W, Grimminger F. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med 2004;141:169-177.
28. Richalet JP, Gratadour P, Robach P, Pham I, Dechaux M, Joncquiert-Latarjet A, Mollard P, Brugniaux J, Cornolo J. Sildenafil inhibits altitude-induced hypoxemia and pulmonary hypertension. Am J Respir Crit Care Med 2005;171:275-281.
29. Maggiorini M, Brunner-La Rocca HP, Bärtsch P, Fischler M, Böhm T, Bloch KE, Mairbäurl H. Dexamethasone and tadalafil prophylaxis prevents both excessive pulmonary constriction and high altitude pulmonary edema in susceptible subjects [abstract]. Eur Respir J 2004; 24:110s.
30. Morganti A, Giussani M, Sala C, Gazzano G, Marana I, Pierini A, Savoia MT, Ohio F, Cogo A, Zanchetti A. Effects of exposure to high altitude on plasma endothelin-1 levels in normal subjects. J Hypertens 1995;13: 859-865.
31. Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol 1998;32:S2-S13.
32. Johnson W, Nohria A, Garrett L, Fang JC, Igo J, Katai M, Ganz P, Creager MA. Contribution of endothelin to pulmonary vascular tone under normoxic and hypoxic conditions. Am J Physiol Heart Circ Physiol 2002;283:H568-H575.
33. Sato K, Rodman DM, McMurtry IF. Hypoxia inhibits increased ETB receptor-mediated NO synthesis in hypertensive rat lungs. Am J Physiol 1999;276:L571-L581.
34. Ivy D, McMurtry IF, Yanagisawa M, Gariepy CE, Le Cras TD, Gebb SA, Morris KG, Wiseman RC, Abman SH. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 2001;280:L1040-L1048.
Marc M. Berger*, Christiane Hesse*, Christoph Dehnert, Heike Siedler, Petra Kleinbongard, Hubert J. Bardenheuer, Malte Kelm, Peter Bärtsch, and Walter E. Haefeli
Departments of Internal Medicine VI (Clinical Pharmacology and Pharmacoepidemiology), Internal Medicine VII (Sports Medicine), and Anesthesiology, University of Heidelberg, Heidelberg; and Department of Medicine, Division of Cardiology, Pulmonary Disease, and Angiology, University of Dusseldorf, Dusseldorf, Germany
(Received in original form April 26, 2005; accepted in final form June 8, 2005)
Supported by grants from the University of Heidelberg, Germany.
* These authors contributed equally to this article.
Correspondence and requests for reprints should be addressed to Walter E. Haefeli, M.D., Department of Internal Medicine VI, Clinical Pharmacology and Pharmacoepidemiology, University of Heidelberg, Im Neuenheimer Feld 410, D-69120 Heidelberg, Germany. E-mail: walter_emil_haefeli@med.uni-heidelberg.de
Am J Respir Crit Care Med Vol 172. pp 763-767, 2005
Originally Published in Press as DOI: 10.1164/rccm.200S04-654OC on June 9, 2005
Internet address: www.atsjournals.org
Copyright American Thoracic Society Sep 15, 2005
Provided by ProQuest Information and Learning Company. All rights Reserved