Phthalates are a group of multifunctional chemicals used in consumer and personal care products, plastics, and medical devices. Laboratory studies show that some phthalates are reproductive and developmental toxicants. Recently, human studies have shown measurable levels of several phthalates in most of the U.S. general population. Despite their widespread use and the consistent toxicologic data on phthalates, information is limited on sources and pathways of human exposure to phthalates. One potential source of exposure is medications. The need for site-specific dosage medications has led to the use of enteric coatings that allow the release of the active ingredients into the small intestine or in the colon. The enteric coatings generally consist of various polymers that contain plasticizers, including triethyl citrate, dibutyl sebacate, and phthalates such as diethyl phthalate (DEP) and dibutyl phthalate (DBP). In this article we report on medications as a potential source of exposure to DBP in a man who took Asacol [active ingredient mesalamine (mesalazine)] for the treatment of ulcerative colitis. In a spot urine sample from this man collected 3 months after he started taking Asacol, the concentration of monobutyl phthalate, a DBP metabolite, was 16,868 ng/mL (6,180 [micro]g/g creatinine). This concentration was more than two orders of magnitude higher than the 95th percentile for males reported in the 1999-2000 National Health and Nutrition Examination Survey (NHANES). The patient's urinary concentrations of monoethyl phthalate (443.7 ng/mL, 162.6 [micro]g/g creatinine), mono-2-ethylhexyl phthalate (3.0 ng/mL, 1.1 [micro]g/g creatinine), and monobenzyl phthalate (9.3 ng/mL, 3.4 [micro]g/g creatinine) were unremarkable compared with the NHANES 1999-2000 values. Before this report, the highest estimated human exposure to DBP was more than two orders of magnitude lower than the no observable adverse effect level from animal studies. Further research is necessary to determine the proportional contribution of medications, as well as personal care and consumer products, to a person's total phthalate burden. Key words: biomarkers, environmental health, medications, phthalates, reproductive health. Environ Health Perspect 112:751-753 (2004). doi:10.1289/ehp.6804 available via http://dx.doi.org/[Online 29 January 2004]
Case Presentation
As part of an ongoing study on environmental agents and male reproductive health, male partners of subfertile couples who presented to the Vincent Burnham Andrology Laboratory at Massachusetts General Hospital (MGH; Boston, MA) provided a semen and a spot urine sample at their clinic visits. Men also completed a questionnaire including information on lifestyle factors, medical history, and medication use. The results of the relationship between phthalates and testicular function have been reported elsewhere (Duty et al. 2003a, 2003b). In this article we report on medication use as a likely source of dibutyl phthalate (DBP) exposure in one of the subjects from this ongoing study who had unusually high urinary concentrations of monobutyl phthalate (MBP), the primary metabolite of DBP.
The study was approved by the Harvard School of Public Health and MGH Human Subjects Committees. Information on medication use was collected with a nurse-administered questionnaire at the time of the visit to the clinic to provide the semen and urine samples. The subject was asked, "Have you recently (past few weeks) taken any medications? If yes, please provide types and date(s) last taken."
Several phthalate monoesters were measured in a single spot urine sample collected in a sterile specimen cup. The analytical approach has been described in detail elsewhere (Blount et al. 2000a; Silva et al. 2003). Briefly, the determination of phthalate metabolites in urine involved enzymatic deconjugation of the metabolites from the glucuronidated form, solid-phase extraction, separation with HPLC, and detection by tandem mass spectrometry. Detection limits were in the low nanogram per milliliter range. [sup.13.][C.sub.4]-labeled internal standards were used to increase the precision of measurements. One method blank, two quality control samples (human urine spiked with phthalates), and two sets of standards were analyzed along with every 21 unknown urine samples. Analysts at the Centers for Disease Control and Prevention (CDC; Atlanta, GA) were blind to all information concerning the subjects. Creatinine adjustment was used to correct for urine dilution.
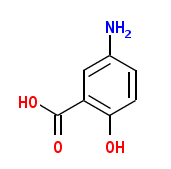
The case patient was in his early thirties and had an unremarkable occupational history, without any known workplace exposures. He had a medical history of ulcerative colitis and reported taking Asacol (Proctor & Gamble, Cincinnati, OH), which contains the active ingredient mesalamine (5-amino-2-hydroxybenzoic acid), also known as 5-ASA or mesalazine in Europe. He took twelve 400 mg Asacol tablets daily during the 3 months before the collection of his urine sample. Although not fully understood, the anti-inflammatory action of mesalamine is thought to be through blocking cyclo-oxygenase and inhibiting prostaglandin production in the colon (Proctor & Gamble Pharmaceuticals 2000; Schroeder 2002). The Asacol delayed-release tablets are coated with methacrylic acid copolymer B (Eudragit-S; Rhom GmbH & Co. KG, Darmstadt, Germany), which dissolves at [greater than or equal to] pH 7, releasing mesalamine in the terminal ileum and beyond for topical anti-inflammatory action in the colon. Other inactive ingredients in Asacol tablets are colloidal silicon dioxide, edible black ink, iron oxide red, iron oxide yellow, lactose, magnesium stearate, polyethylene glycol, povidone, sodium starch glycolate, talc, and DBP (Proctor & Gamble Pharmaceuticals 2000).
The patient's urinary MBP concentration was 16,868 ng/mL (6,180 [micro]g/g creatinine), whereas monoethyl phthalate (MEP), mono-2-ethylhexyl phthalate (MEHP), and monobenzyl phthalate (MBzP) concentrations were 443.7 ng/mL (162.6 [micro]g/g creatinine), 3.0 ng/mL (1.1 [micro]/g creatinine), and 9.3 ng/mL (3.4 [micro]g/g creatinine), respectively.
Discussion
There is scientific and public concern about potential human health risks from exposure to phthalates, diesters of phthalic acid. These concerns stem from studies showing that a large proportion of the U.S. general population are exposed to phthalates (Blount et al. 2000b; CDC 2003), as well as from animal studies consistently showing that some phthalates are developmental and reproductive toxicants (Agarwal et al. 1985; Cater et al. 1977; Foster et al. 1980; Mylchreest et al. 1999, 2000; Park et al. 2002; Sjoberg et al. 1986). Two recent reports on the levels of phthalate monoester metabolites in urine samples collected for the third U.S. National Health and Nutrition Examination Survey (NHANES III) (Blount et al. 2000a) and NHANES 1999-2000 (CDC 2003) showed that four phthalate metabolites, MEP, MEHP, MBP, and MBzP, were present in more than 75% of subjects sampled. Monoester phthalate metabolites were measured because of potential sample contamination from the parent diester and because the metabolites are considered the biologically active toxicant (Li et al. 1998; Peck and Albro 1982). The preferred measure of phthalate exposure is urinary phthalate monoester metabolite levels because they represent an integrative measure of exposure to phthalates from the multiple sources and routes of exposure (CDC 2003; Silva et al. 2003).
Phthalates are a family of multifunctional chemicals that are used in countless and diverse products. They are used to hold color and scent in consumer and personal care products; as solvents in paints, glue, insect repellents, lubricants, and adhesives; and to soften a wide range of plastics, including medical products such as polyvinyl chloride (PVC) blood products and intravenous bags, as well as dialysate bags and tubing [Agency for Toxic Substances and Disease Registry (ATSDR) 1995, 2000, 2001; Nassberger et al. 1987]. Diethyl phthalate (DEP), dibutyl phthalate (DBP), and butyl benzyl phthalate (BBzP) are principally used in personal care products, such as body lotions, gels, shampoos, and deodorants (ATSDR 1995, 2001). Several phthalates, including DBP, have U.S. Food and Drug Administration (FDA) approval as inert ingredients in medications and as indirect food additives for uses as adhesives and components of coatings in food packaging and processing materials that are in contact with food (U.S. FDA 2000, 2003). As a result, phthalates may be ingested in medications and foods (Castle et al. 1990; Page and Lacroix 1995). Phthalates such as DBP, BBzP, and di-2-ethylhexyl phthalate are also used in residential building materials such as floorings, paints, carpet backings, adhesives, and wallpaper and in PVC products such as auto parts and interiors (ATSDR 1995, 2000). Although the volatility of phthalates is relatively low, studies have shown that phthalates are present in residential indoor air (Rudel et al. 2003).
Human exposure to phthalates can occur via ingestion, inhalation, and dermal routes, as well as through parenteral exposure from medical devices containing phthalates. Currently, no human data on the proportional contribution of the various sources of phthalates to human body burden are available. Until recently, the primary exposure of the general population to phthalates was believed to result from ingestion of foods, especially fatty foods such as milk, butter, and meats. Recent data show that the low-molecular-weight phthalates (DEP, DBP, BBzP) may also be dermally absorbed and that the more volatile phthalates can be inhaled (ATSDR 1995, 2001).
The need for site-specific dosage medications has led to the use of enteric coatings (Ashford and Fell 1994; Marvola et al. 1999). Enteric-coated medications remain intact in the stomach and release the active ingredients of the underlying medication core into the lower part of the small intestine or in the colon. Enteric coatings generally consist of various polymers, such as cellulose acetate phthalate, cellulose acetate butyrate, ethylcellulose, polyvinyl acetate phthalate, and methacrylate copolymers. These polymer coatings are plasticized with compounds such as triethyl citrate, dibutyl sebacate, and phthalates such as DEP and DBP (Frohoff-Hulsmann et al. 1999; Harris and Ghebre-Sellassie 1989). DEP and DBP metabolize to MEP and MBP, respectively, which can be measured in urine. In addition to tablets and capsules, other medications and preparations may contain phthalates as plasticizers. Specifically, cellulose acetate--free films for transdermal use may contain DBP as a plasticizer (Rao and Diwan 1997).
The patient's urinary phthalate concentrations were compared with the concentrations measured for the Second National Report on Human Exposure to Environmental Chemicals (CDC 2003; Table 1). CDC (2003) reported data on phthalate monoester levels from participants in NHANES 1999-2000. NHANES is an ongoing survey designed to measure the health and nutrition status of the civilian non-institutionalized U.S. population [National Center for Health Statistics (NCHS) 2003]. The samples in the present study and the NHANES 1999-2000 samples were analyzed by the same CDC laboratory.
Compared with the NHANES 1999-2000 data set, the patient's urinary MEP, MEHP, and MBzP levels were unremarkable. However, the patient's concentration of MBP in urine was two orders of magnitude higher than the U.S. population 95th percentile for males reported in the NHANES 1999-2000 data set. To date, the highest estimated DBP exposure in a subset of the U.S. population, specifically women of childbearing age (Kohn et al. 2000), was considered more than two orders of magnitude lower than the lowest no observable adverse effect level (NOAEL) of DBP from animal studies (Mylchreest et al. 2002). Our results suggest that medications taken chronically can contribute to DBP exposure that approaches the NOAEL measured in animal studies. Further research is necessary to determine whether any health risks are associated with human exposure to high levels of MBP.
Our results suggest that in some circumstances individuals may be highly exposed to phthalates. Therefore, in epidemiologic studies designed to determine predictors of exposure to phthalates or potential health risks of phthalates, a detailed assessment of medication use is important. Because phthalates may be used in a variety of medications, a complete record of all medications used and the date the prescription was filled are needed to begin to describe human exposure. The list of medications should include medications taken by all routes, including orally, nasally, subgingivally, ophthalmologically, dermally, intravenously, or by inhalation.
Conclusion
In the present study, we identified an individual with a urinary MBP level two orders of magnitude higher than the U.S. population 95th percentile and linked this unusually high urinary MBP concentration with the use of a specific medication that contained DBP. However, because this is a case report on a single patient, replication of this finding in other populations is needed to definitively conclude that the medication was the main contributor to the very high urinary concentration of MBP. Additionally, further research is needed to determine the proportional contribution of medications, as well as other consumer products, to a person's total phthalate burden. Adding to the complexity of apportioning sources of phthalates is that individuals may be exposed orally as well as by inhalation or dermal routes. When databases on consumer products and foods containing phthalates become available, these databases should be linked with levels of urinary phthalates measured in ongoing epidemiologic investigations. Until this time, individual case reports such as this one will provide clues on relevant sources of human exposure and their potential contribution to total body burden.
REFERENCES
Agarwal DK, Maronpot RR, Lamb JC, Kluwe WM. 1985. Adverse effects of butyl benzyl phthalate on the reproductive and hematpoietic systems of male rats Toxicology 35:189-206.
Ashford M, Fell J. 1994. Targeting drugs to the colon: delivery systems for oral administration. J Drug Target 2:241-258.
ATSDR. 1995. Toxicological Profile for Diethyl Phthalate. Atlanta, GA:Agency for Toxic Substances and Disease Registry.
ATSDR. 2000. Toxicological Profile for Di-2-(ethylhexyl) Phthalate. Atlanta, GA:Agency for Toxic Substances and Disease Registry.
ATSDR. 2001 Toxicological Profile for Di-n-butyl Phthalate. Atlanta, GA:Agency for Toxic Substances and Disease Registry.
Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, et at 2000b. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem 72:4127-4134.
Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, et al. 2000a. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect 108:979-982.
Castle L, Jickells SM, Gilbert J, Harrison N. 1990. Migration testing of plastics and microwave-active materials for high-temperature food-use applications. Food Addit Contam 7:779-796.
Cater BR, Cook MW, Gangolli SD, Grasso P. 1997. Studies on dibutyl phthalate-induced testicular atrophy in the rat: effect on zinc metabolism. Toxicol Appl Pharmacol 41:609-618.
CDC. 2003. Second National Report on Human Exposure to Environmental Chemicals. NCEH Pub. No. 02-0716, Atlanta, GA:Centers for Disease Control and Prevention, National Center for Environmental Health. Available: http:// www.cdc.gov/exposurereport [accessed 6 October 2003].
Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, et al. 2003a. The relationship between environmental exposure to phthalates and human semen parameters. Epidemiology 14:209-227.
Duty SM, Singh NP, Silva MJ, Barr DB, Brock JW, Ryan L, et al. 2003b. The relationship between environmental exposure to phthalates and DNA damage in human sperm using the neutral comet assay, Environ Health Perspect 111:1164-1169.
Foster PMD, Thomas LV, Cook MW, Gangolli SD. 1980. Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicol Appl Pharmacol 54:392-398.
Frohoff-Hulsmann MA, Schmitz A, Lippold BC. 1999. Aqueous ethyl cellulose dispersions containing plasticizers of different water solubility and hydroxypropyl methylcellulose as coating material for diffusion pellets I. Drug release rates from coated pellets. Int J Pharm 177:69-82.
Harris MR, Ghebre-Sellassie I. 1989. Aqueous polymeric coatings for modified-release pellets. In: Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms (McGinity JW, ed). New York:Marcel Dekker, 81-100.
Kohn MC, Parham F, Masten SA, Portier CJ, Shelby MD, Brock JW, et at. 2000, Human exposure estimates for phthalates. Environ Health Perspect 108:440-442.
Li L-H, Jester WF, Orth JM. 1998. Effects of relatively low levels of mono-(2-ethylhexyl) phthalate on cocultured sertoli cells and gonocytes from neonatal rats. Toxicol Appl Pharmacol 153:253-265.
Marvola M, Nykanen P, Rautio S, Isonen N, Autere A-M. 1999. Enteric polymers as binders and coating materials in multiple-unit site-specific drug delivery systems. Eur J Pharm Sci 7:259-267.
Mylchreest E, Madhabananda S, Sar M, Cattley RC, Foster PMD. 1999. Disruption of androgen regulated male reproductive development by Di(n-butyl)phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol 156:81-95.
Mylchreest E, Sar M, Wallace DG, Foster PMD. 2002. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol 16:19-28.
Mylchreest E, Wallace DG, Cattley RC, Foster PMD. 2000. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol Sci 55:143-51.
Nassberger L, Arbin A, Ostelius J. 1987. Exposure of patients to phthalates from polyvinyl chloride tubes and bags during dialysis. Nephron 45:280-290.
NCHS. 2003. National Health and Nutrition Examination Survey. Hyattsville, MD:National Center for Health Statistics. Available: http://www.cdc.gov/nchs/nhanes.htm [accessed 11 August 2003].
Page BD, Lacroix GM. 1995. The occurrence of phthalate esters and di-2-ethylhexyl adipate plasticizers in Canadian packaging and food samples in 1985-1989: a survey, Food Addit Contam 12:129-151.
Park JD, Habeebu SSM, Klaassen CD. 2002. Testicular toxicity of di-(2-ethylhexyl)phthalate in young Sprague-Dawley rats. Toxicology 171:103-115.
Peck CC, Albro PW, 1982. Toxic potential for the plasticizer di(2-ethylhexyl) phthalate in the context of its disposition and metabolism in primates and man. Environ Health Perspect 45:11-17.
Proctor & Gamble Pharmaceuticals. 2000. Asacol (Mesalamine) Delayed Release Tablets. Cincinnati, OH:Proctor and Gamble Pharmaceuticals, Available: http://www.pgpharma.com/ pi/US-Asacol.pdf [accessed 10 May 2003].
Rao PR, Diwan PV. 1997. Permeability studies of cellulose acetate free films for transdermal use: influence of plasticizers Pharm Acta Helv 72:47-51.
Rudel RA, Camann DE, Spongier JD, Korn LR, Brody JG. 2003. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37:4543-4553.
Schroeder KW. 2002. Role of mesalazine in acute and long-term treatment of ulcerative colitis and its complications. Scand J Gastroenterol Suppl 236:42-47.
Silva MJ, Malek NA, Hodge CC, Reidy JA, Kate K, Barr DB, et al. 2003. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B 789:393-404.
Sjoberg P, Lindquist NG, Ploen L. 1986. Age-dependent response of the rat testis to di-2-(ethylhexyl) phthalate. Environ Health Perspect 65:237-242.
U.S. FDA (U.S. Food and Drug Administration). 2000. Indirect Food Additives: Adhesives and Components of Coatings: Adhesives. 21 CFR 175.105. Available: http://www. access.gpo.gov/nara/cfr/waisidx_00/21cfr175_00.html [accessed 5 January 2004].
U.S. FDA (U.S. Food and Drug Administration). 2003. Inactive Ingredient Search for Approved Drug Products. Rockville, MD:U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Available: http://www.access-data.fda.gov/scripts/cder/iig/index.cfm [accessed 5 January 2004].
Russ Hauser, (1,2) Susan Duty, (1) Linda Godfrey-Barley, (1) and Antonia M. Calafat (3)
(1) Department of Environmental Health, Occupational Health Program, Harvard School of Public Health, Boston, Massachusetts, USA; (2) Vincent Memorial Obstetrics and Gynecology Service, Andrology Laboratory and In Vitro Fertilization Unit, Massachusetts General Hospital, Boston, Massachusetts, USA; (3) National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA
Address correspondence to R. Hauser, Occupational Health Program, Harvard School of Public Health, Building l, Room 1405, 665 Huntington Ave., Boston, MA 02115 USA. Telephone: (617) 432-3326. Fax: (617) 432-0219. E-mail: rhauser@ hohp.harvard.edu
We thank M. Silva for the chemical analysis.
This study was supported by grants ES09718 and ES00002 from the National Institute of Environmental Health Sciences.
The authors declare they have no competing financial interests.
Received 16 October 2003; accepted 27 January 2004.
COPYRIGHT 2004 National Institute of Environmental Health Sciences
COPYRIGHT 2004 Gale Group