Glue? Maybe. Stew? Definitely. But it's hard to imagine the eastern oyster having anything to do with detergents and disposable diapers. Those two commercial applications are in the works, however, inspired by a study of how the oyster makes its shell.
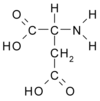
Like bones and teeth, oyster shell is a structure of mineralized calcium bound with protein. Dissolve the calcium, and what's left is a gel. In the early 1980s, biologist Hap Wheeler of Clemson (S.C.) University found the gel to be a mixture of large and small proteins containing chains, or polymers, of an amino acid called aspartic acid. It's an unusual amino acid because it holds a negative charge-a particularly important property when the amino acid is linked into a polymer, says Wheeler. He spoke at a marine biotechnology briefing in Washington, D.C., earlier this month.
In the oyster, proteins that bristle with negatively charged polyaspartic acid control the growth of the shell. They attract the positively charged calcium and hold it in place. Manufacturers add similar polyanions-agents with many negative charges-to detergents to attract and hold particles of dirt. The dirt then stays suspended in the wash water "instead of landing on your clothes," says Wheeler.
Existing polymers used in laundry and dishwashing detergents and other applications don't break down easily. Polyacrylic acid, for example, has a hard-to-degrade carbon-carbon backbone. In laboratory tests, however, bacteria attacked the amino acid bonds of the oyster polymer; field tests are now underway.
"The polymer is biodegradable," declares Wheeler. That's why the oyster-inspired polymer spawned the start-up of the Donlar Corp. in Bedford Park, Ill., in 1990. The company does not harvest oyster shells for production. Instead, it begins with powdered aspartic acid, which is heated and modified to form the polymer (SN: 7/13/96, p. 22).
The oyster shell proteins also absorb water. Wheeler says this property may make the shell less brittle in the same way that collagen strengthens bone. He and his colleagues have found that polyaspartic acid can absorb up to 80 times its weight in water, making it at least modestly competitive with the nondegradable superabsorbent gels now used in disposable diapers and other products.
The search for biodegradable polymers is attracting much commercial interest, says biochemist Barry Marrs of Kennett Square, Pa., who is on an industrial biotechnology task force for the United Nations' Organization for Economic Cooperation and Development, headquartered in Paris.
"Polyaspartic acids are very nicely functional," he says, although he points out that Donlar's industrial version is not the same as the oyster's and probably is not as biodegradable. "But I do believe it is an advance" over the nondegradable commercial polymers widely used for detergents and absorbents. Hundreds of millions of tons of them find their way into household products each year, according to Wheeler.
While developing uses for its polymer in detergents and superabsorbents, Donlar began marketing it for another use-as a fertilizer additive. The polymer seems to enhance plant growth, presumably by attracting positively charged soil nutrients that the roots absorb. Polyaspartic acid has also been put to work in controlling corrosion (SN: 5/4/91, p. 287). - C.M.
COPYRIGHT 1997 Science Service, Inc.
COPYRIGHT 2004 Gale Group