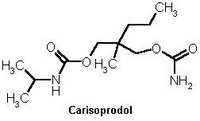
Ranbaxy Pharmaceuticals will market carisoprodol 350 mg tablets in bottles of 100, 500 and 1,000. Core Pharma was granted FDA approval for carisorodol. Under a strategic alliance formed between Ranbaxy and Core, the product will be marketed under the Ranbaxy label and promoted through the Ranbaxy Pharmaceuticals sales force. Carisoprodol is indicated as an adjunct to rest, physical therapy and other measures for the relief of discomfort associated with acute, painful muscular skeletal conditions.
COPYRIGHT 2000 Lebhar-Friedman, Inc.
COPYRIGHT 2001 Gale Group