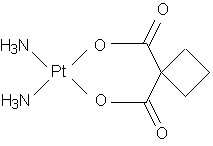
Carboplatin
Carboplatin is a chemotherapy drug used against some form of cancer. It was introduced in the late 1980s and has since gained popularity in clinical treatment due to its vastly reduced side-effects compared to its parent compound cisplatin. Cisplatin and carboplatin, as well as oxaliplatin, are classified as DNA alkylating agents. more...
History
Bristol-Myers Squibb gained FDA approval for carboplatin, under the brand name Paraplatin, in March 1989. The drug went generic in October 2004.
Suppliers
Bristol-Myers Squibb continues to market Paraplatin. There are also generic versions of the drug available from APP, Bedford, Sicor, Mayne Pharma, Pharmachemie, Pliva, Sandoz, Spectrum.
Pharmacology
Chemistry
Carboplatin differs from cisplatin in that it has a closed cyclobutane dicarboxylate (CBDCA) moiety on its leaving arm in contrast to the readily leaving chloro groups. This results in very different DNA binding kinetics, though it forms the same reaction products in vitro at equivalent doses with cisplatin. However, recent studies provide a new caveat on the DNA binding molecular mechanisms with the possibility of being activated by nucleophiles (as opposed to cisplatin), before forming the toxic adducts. There are also results to show that cisplatin and carboplatin cause different morphological changes in MCF-7 cell lines while exerting their cytotoxic behaviour.
Mode of action
Two theories exist to explain the molecular mechanism of action of carboplatin with DNA.
- Aquation, or the like-cisplatin hypothesis.
- Activation, or the unlike-cisplatin hypothesis.
The former is more accepted owing to the similarity of the leaving groups with its predecessor cisplatin, while the latter hypothesis envisages a biologically activation mechanism to release the active Pt2+ species.
Side-effects
The largest benefit of using carboplatin over cisplatin is the reduction of side effects; particularly the elimination of cisplatin's nephrotoxic effects. This is due in part to the added stability of carboplatin in the bloodstream, which prevents proteins from binding to it. This in turn reduces the amount of these protein-carboplatin complexes to be excreted. The lower excretion rate of carboplatin means that more is retained in the body, and hence its effects are longer lasting (a retention half-life of 30 hours for carboplatin, compared to 1.5-3.6 hours in the case of cisplatin).
There are no known ototoxic effects from carboplatin. Nausea and vomiting are less severe and more easily controlled, compared to the incessant vomiting and antiperistalsis that some patients using cisplatin may experience. Carboplatin has also proven effective in some strains of cancer that may not be susceptible to cisplatin, including germ-line cell, small and non-small cell lung, ovary, and bladder cancers, as well as acute leukemia.
The main drawback of carboplatin is its myelosuppressive effects. This causes the blood cell and platelet output of bone marrow in the body to decrease quite dramatically, sometimes as low as 10% of its usual production levels. The nadir of this myelosuppression usually occurs 21-28 days after the first treatment, after which the blood cell and platelet levels in the blood begin to stabilize, often coming close to its pre-carboplatin levels. This decrease in white blood cells (neutropenia) causes many complications, most notably infection by opportunistic organisms. This necessitates readmission to hospital and treatment with antibiotics.
Read more at Wikipedia.org