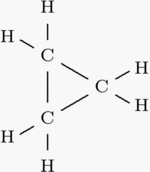
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6 consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms. The bonds between the carbon atoms are a great deal weaker than in a typical carbon-carbon bond. This is the result of the 60° angle between the carbon atoms, which is far less than the normal angle of 109.5°. more...
This ring strain has to be subtracted from the normal C-C bond energy, making the resultant compound more reactive than acyclic alkanes and other cycloalkanes such as cyclohexane and cyclopentane. This is the banana bond description of cycloalkanes.
However, cyclopropanes are more stable than a simple angle strain analysis would suggest. This is because the banana-bond model (without major tweaks) is incorrect; cyclopropane is better modeled as a three-center-bonded orbital combination of methylene carbenes. This results in the walsh orbital description of cyclopropane, where the C-C bonds have mostly pi character. This is also why cyclopropanes often have reactivity similar to alkenes. This is also why carbenes can easily add into alkenes to produce cyclopropanes.
Boiling point: -32.8°C Freezing point: -127°C
The naturally occurring pyrethrum insecticides (found in certain Chrysanthemum species) contain a cyclopropane ring.
Cyclopropane is an anaesthetic when inhaled, but has been superseded by other agents in modern anaesthetic practice. This is due to its extreme reactivity under normal conditions: when the gas is mixed with oxygen there is a significant risk of explosion.
Because of the strain in the carbon-carbon bonds of cyclopropane, the molecule has an enormous amount of potential energy. In pure form, it will break down to form linear alkanes and alkenes, including "normal", non-cyclic propane. This decomposition is potentially explosive, especially if the cyclopropane is liquified, pressurized, or contained within tanks. Explosions of cyclopropane and oxygen are even more powerful, because the energy released by the formation of normal propane is compounded by the energy released via the oxidation of the carbon and hydrogen present. At room temperature, sufficent volumes of liquified cyclopropane will self-detonate. To gaurd against this, the liquid is shipped in cylinders filled with tungsten wool, which prevents high-speed collisions between molecules and vastly improves stability. Pipes to carry cyclopropane must likewise be of small diameter, or else filled with unreactive metal or glass wool, to prevent explosions. Even if these precautions are followed, cyclopropane is dangerous to handle and manufacture, and is no longer used for anaesthesia.
Read more at Wikipedia.org