Capoten
Captopril is an Angiotensin-Converting Enzyme inhibitor (ACE inhibitor) used for the treatment of hypertension and some types of chronic heart failure. Captopril was the first ACE inhibitor developed and was considered a breakthrough both because of its novel mechanism of action and also because of the revolutionary development process. The original innovator drug Bristol-Myers Squibb's Capoten®. more...
Clinical Use
Development of captopril
Captopril was invented in 1975 by three researchers at the American drug company Squibb (now Bristol-Myers Squibb) , Miguel Ondetti, Bernard Rubin and David Cushman. Squibb filed for American patent protection on the drug in February 1976 and U.S. Patent was 4,046,889 was granted in September 1977.
The development of captopril was amongst the earliest successes of the revolutionary concept of structure-based drug design. The renin-angiontensin-aldosterone system had been extensively studied in the mid-20th century and it had been decided that this system presented several opportune targets in the development of novel treatments for hypertension. The first two targets that were attempted were renin and ACE. Captopril was the culmination of efforts by Squibb's laboratories to develop an ACE inhibitor.
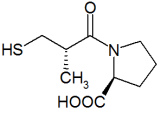
Ondetti, Cushman and colleagues built on work that had been done in the 1960s by the British Nobel laureate Sir John Vane when he was a researcher at the Royal College of Surgeons. Working with a Brazilian colleage, Sérgio Ferreira, Vane discovered a peptide in Brazilian viper venom which was a 'collected-product inhibitor' of angiotensin II. Captopril was developed from this peptide after it was found via QSAR-based modification that the terminal sulfhydryl-moiety of the peptide provided a high potency of ACE inhibition.
Capoten gained FDA approval in June 1981. The drug went generic in the U.S. in February 1996 as a result of the end of market exclusivity for Bristol-Myers Squibb.
Shortcomings
During Phase III/IV trials of captopril, it was found that captopril had some undesirable adverse effects. The most predominant of which included cough, rash and taste disturbances (metallic or loss of taste). Cough is an adverse effect common to all of the ACE inhibitors, but the rash and taste disturbances were attributed to the very sulfhydryl moiety which granted captopril its potency. An additional shortcoming of captopril is the short half-life, necessitating 2-3 times daily dosing.
The development of longer-acting ACE inhibitors lacking the sulfhydryl-moiety such as enalapril proved to be the downfall of captopril and, whilst it is still used, it is no longer amongst the more widely used ACE inhibitors.
Reference
- Smith CG, Vane JR. The discovery of captopril. FASEB J 2003;17:788-9. Fulltext. PMID 12724335.
Read more at Wikipedia.org