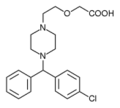
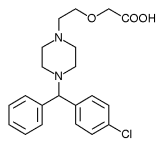
Pfizer and UCB Pharma have received FDA approval of chewable cetirizine (Zyrtec) for the treatment of seasonal and perennial allergic rhinitis and chronic idiopathic urticaria in children aged 2 years and older.
The chewable formulation of cetirizine offers 24-hour symptom relief and will be available in 5 mg and 10 mg doses in a grape-flavored tablet. It can be taken with or without food or water.
Cetirizine chewable tablets are bioequivalent to cetirizine tablets and offer the same efficacy as cetirizine tablets and syrup.
Most side effects associated with cetirizine are mild or moderate. In infants, side effects associated with cetirizine are similar to placebo and include crankiness, fussiness, and insomnia. Side effects in children aged 2 to 11 years include drowsiness, headache, sore throat, and stomach ache.
Copyright Springhouse Corporation Jun 2004
Provided by ProQuest Information and Learning Company. All rights Reserved